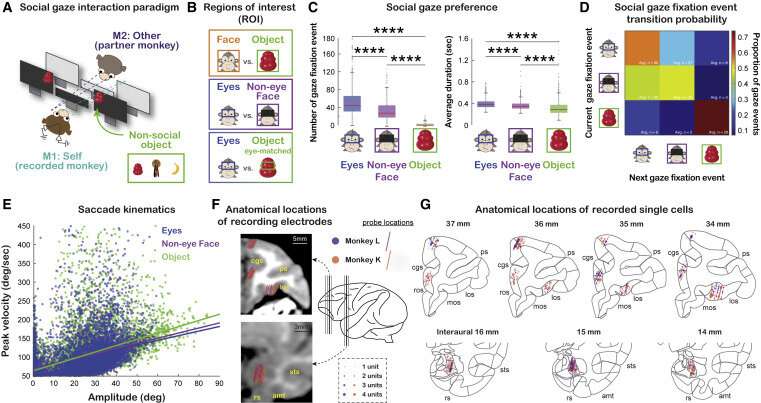
Experimental setup, social gaze behaviors, and recording sites. (A) Experimental paradigm for studying naturalistic, face-to-face, social gaze interaction. The inset shows three different types of non-social objects used. (B) Illustrations of the gaze ROIs and contrasts.(C) Social gaze preference, indicated as the total number of fixations and the average duration per fixation to eyes, non-eye face, and object. ∗∗∗∗p < 0.0001, Wilcoxon sign rank, two sided, FDR corrected.(D) Social gaze fixation event transition probability for pairs of current and next fixations to eyes, non-eye face, and object. The number in each cell shows the average frequency of a particular transition across days.(E) Saccade kinematics quantified by peak velocity and amplitude for all saccades to eyes (blue), non-eye face (purple), and object (green) (lines, linear regression).(F) Anatomical locations of electrode positions from monkey L (purple) and monkey K (orange) on representative coronal MRI slices of monkey K (black thick lines in the brain illustration) (cgs, cingulate sulcus; ps, principal sulcus; los, lateral orbitofrontal sulcus; sts, superior temporal sulcus; amt, anterior middle temporal sulcus; rs, rhinal sulcus).(G) Anatomical locations of recorded single cells in OFC, dmPFC, ACCg, and BLA. Recording locations from monkey L and monkey K are projected onto the standard stereotaxic coordinates of the rhesus macaque brain atlas. Four representative coronal slices with 1-mm interaural spacing were chosen for the three prefrontal areas and three representative coronal slices were chosen for BLA (same as the slices indicated in F). Credit: Neuron (2022). DOI: 10.1016/j.neuron.2022.04.013
Their eyes met across a crowded dance floor, causing specialized neurons to begin firing in multiple regions of both brains that are tasked with deriving meaning from a social gaze.
Although not as romantic as the first dance floor encounter, a new Yale study was able to chart this surprisingly widespread neuronal response in multiple brain areas when the eyes of two individuals meet and social gaze interaction happens, researchers report May 10 in the journal Neuron.
"There are strong robust signals in the brain that are signatures of an interactive social gaze," said Yale's Steve Chang, associate professor of psychology and neuroscience, a member of the Wu-Tsai Institute and the Kavli Institute for Neuroscience, and the senior author of the study.
The phenomenon of extracting meaning in the gaze between two people has been documented in art and literature for millennia but scientists have had a difficult time uncovering how the brain accomplishes such a subtle feat. They have extensively studied the neurobiology of social perception, usually by giving brain scans to individuals as they are presented with specific static images, such as angry or happy faces or direct or averted gazes. However, the interactions of two individual minds as they dynamically and reciprocally extract information from each other's eyes are difficult to tackle.
Chang's lab overcame this obstacle by monitoring the brain activity of monkeys while simultaneously tracking the eye positions of two animals. This enabled them to record a large array of neurons as the animals spontaneously gazed at each other.
"They were spontaneously engaging in social interactions while we examined neural firing," Chang said. "Importantly, we were not imposing any tasks, so it was up to them to decide how and when they would interact."
They found that specific sets of socially tuned neurons fired across multiple brain regions at different times during mutual eye contact. For instance, one set of neurons fired when one individual initiated mutual eye contact, but not when that individual followed the other's gaze. Another set of neurons were active when the monkeys were in the process of deciding whether to complete mutual eye contact initiated by the other. And interestingly, when fixing a gaze onto another individual some neurons marked the distance relative to another's eyes, but when receiving a gaze yet another set of neurons signaled how close the other individual was.
The brain regions in which neuronal activation took place provided hints into how the brain assesses the meaning of the gaze. Surprisingly, part of the network activated during social gaze interaction included the prefrontal cortex, the seat of higher-order learning and decision-making, as well as the amygdala, the center for emotion and valuation.
"Multiple regions within the prefrontal cortex, in addition to the amygdala, are recruited to compute selective aspects of interactive social gaze, suggesting the importance of a more contemplative role during social gaze interaction," Chang said.
These areas in the prefrontal-amygdala networks activated during the processing of social gaze interaction are also known to be disrupted in cases of atypical social conditions, such as autism. This attests to their importance in achieving feelings of social connectedness, he said. Social gaze interaction likely serves a critical role in shaping social connectedness, he added, and the prefrontal-amygdala networks might make that happen.
"The fact that interactive social gaze neurons are found widely in the brain also speaks to the ethological importance of social gaze interaction," Chang said.
Yale's Siqi Fan and Olga Dal Monte are co-lead authors of the study.
More information: Olga Dal Monte et al, Widespread implementations of interactive social gaze neurons in the primate prefrontal-amygdala networks, Neuron (2022). DOI: 10.1016/j.neuron.2022.04.013
Journal information: Neuron
Provided by Yale University