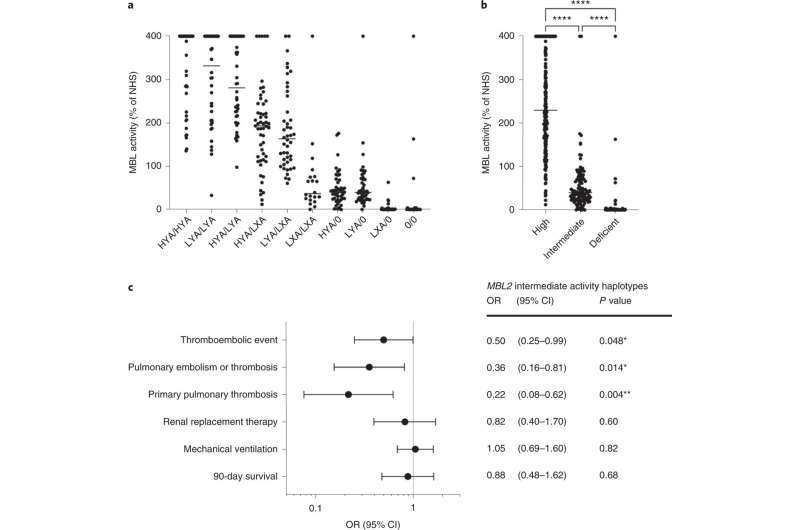
MBL2 haplotypes predict MBL activity in plasma and thromboembolic complications during intensive care. a, MBL activity across individual haplotype combinations. MBL activity in plasma at day 1 of intensive care was measured by a functional ELISA using a mannan matrix for MBL capture. Activity is expressed as a percentage of pooled normal human serum (NHS). The genetic variants shown in Table 1, rows 1–5 give rise to six major MBL2 haplotypes, which are denoted by their legacy names: HYA, LYA, LXA, HYD, LYB and LYC. The HYA, LYA and LXA haplotypes encode wild-type MBL protein and are referred to as 'A' haplotypes. The HYD, LYB and LYC haplotypes contain the missense variants referred to as the D, B or C alleles, respectively, and are shown together as '0' haplotypes because they share a common deleterious effect on MBL activity. b, Haplotypes categorized according to MBL activity into high (HYA/HYA, LYA/LYA, HYA/LYA, HYA/LXA, LYA/LXA), intermediate (LXA/LXA, HYA/0, LYA/0) and deficient (LXA/0, 0/0). MBL activity differed significantly between the groups (230% (168–400, median and interquartile range) for the high group, 40% (24–70) for the intermediate group and 0.56% (0.00–1.25) for the deficient group; P < 0.0001). The horizontal bars indicate the median. c, Forest plot displaying ORs with 95% confidence intervals (CIs) for thromboembolic events, renal replacement therapy, need for mechanical ventilation and 90-day survival. Primary pulmonary thrombosis comprises events without a known concurrent distal venous thrombosis. A logistic regression model with MBL2 intermediate activity haplotypes, sex and age as covariates was used. *P < 0.05, **P < 0.01, **** P < 0.0001. Credit: Nature Immunology (2022). DOI: 10.1038/s41590-022-01227-w
A gene variant in the natural immune system influences the risk for blood clots in the lungs of severely ill COVID-19 patients. This has been documented in a new study by researchers at Uppsala University and Karolinska Institutet that has now been published in Nature Immunology.
"With this study, we are a step closer in explaining why COVID-19 patients have a substantially increased risk of blood clots," says Oskar Eriksson, medical doctor and researcher at Uppsala University and one of the article's main authors.
It became apparent during the pandemic that patients with COVID-19 had a substantial increased risk of suffering blood clots, even when treated with blood thinners. Why COVID-19 leads to such a major activation of the blood's coagulation system in some patients is not yet clear. Researchers at Uppsala University and Karolinska Institutet have now discovered that a gene variant, which regulates the levels of the protein mannose-binding lectin in the blood, protects against blood clots in the lungs.
Recognizes the coronavirus
Mannose-binding lectin is part of the natural immune system. It discovers and eliminates cells infected by bacteria and viruses. Another recent study (Stravalaci et al. Nature Immunology volume 23, pages 275–286 (2022)) showed that mannose-binding lectin recognizes the SARS-CoV-2 virus that causes COVID-19 and can neutralize cells infected with the virus.
"We can now show how mannose-binding lectin influences the course of the illness COVID-19. It is striking that it is the risk for blood clots that is impacted, and we could not see that mannose-binding lectin influences the risk of becoming ill with COVID-19. We can show that the immune system is important for blood clot formation in COVID-19 patients, something that was long suspected and that can explain why such high doses of blood thinners have been necessary," says Oskar Eriksson.
"The large studies that have been presented in the last year have identified several gene variants that influence the risk of becoming severely ill from COVID-19. Our work shows that there is also a genetic background for which complications affect COVID-19 patients," says Hugo Zeberg, medical doctor and researcher at Karolinska Institutet and co-responsible for the study.
More information: Michael Hultström et al, Genetic determinants of mannose-binding lectin activity predispose to thromboembolic complications in critical COVID-19, Nature Immunology (2022). DOI: 10.1038/s41590-022-01227-w
Journal information: Nature Immunology
Provided by Uppsala University