The 'mutational signatures' of many genes hold the key to better cancer therapies
As an approach to personalized medicine, a new Nature Communications study proposes that "mutational footprints" of DNA repair are a promising predictive genetic marker for determining which tumors will respond to certain therapies.
Cancer therapy increasingly relies on a personalized approach, where genetic changes in an individual tumor can be used to determine the best therapeutic strategy. In many cases thus far, these genetic changes included a so-called driver mutation that would predict response to a drug. For instance, mutations in the BRAF gene in melanoma predict response to BRAF inhibitor drugs, and amplifications in the ERBB2 gene in breast cancer predict response to ERBB inhibitor drugs.
However, these examples of successful drug markers are still quite rare. For many mutated driver genes, specific drugs to target them are not available. Moreover, tumors of different patients show high variability in response to drugs and such variability is often not linked to driver gene mutations.
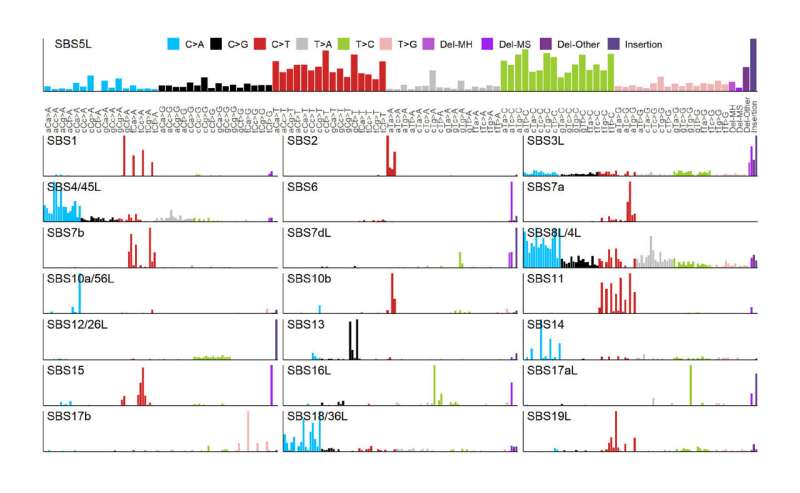
Researchers at IRB Barcelona, led by Dr. Fran Supek, ICREA researcher and head of the Genome Data Science lab, have found that so-called mutational signatures can accurately predict the activity of various drugs applied to cancer cells originating from many types of tumors. These mutational signatures do not originate from driver genes; instead, they reflect a collection of mutations found across the entire genome of a tumor. The mutational signatures can reflect, for example, that the tumor has difficulties in copying or repairing DNA, which may make it more amenable to therapy.
"We have performed statistical analysis using machine-learning methods, considering jointly cancer cell genomes, their response to various drugs, and their response to gene editing experiments. Surprisingly, our analysis revealed that the 'classical' genetic markers such as driver gene mutations or copy-number changes are often less powerful than the mutational signature genetic markers in predicting drug response," explains Dr. Supek.
DNA repair deficiencies make cancer cells easier to target by many drugs
This study found many statistical predictions linking an observed mutational signature with the response (or lack of) towards a cancer drug. It was previously known that a certain type of deficiency in so-called BRCA genes—which can cause breast, ovarian and prostate cancers—predicts response to drugs targeting BRCA deficiency. This deficiency also leaves a mutational signature in the genome of certain types of deletions (removed DNA), which can signal that the tumor is treatable by drugs targeting BRCA deficiency.
In the current study, IRB Barcelona researchers, headed by the Marie Curie postdoctoral fellow Dr. Jurica Levatić, now a postdoctoral researcher in the Jozef Stefan Institute in Slovenia, have shown that this is only one example of many: various types of DNA repair deficiency, such as defects in the "genomic spellchecker" (DNA mismatch repair), can predispose cancer cells to sensitivity to certain drugs. Given that tumors have impaired DNA repair mechanisms, these predicted therapies would have a greater capacity to kill cancer cells and spare healthy ones.
Prior exposure to mutagenic chemicals, including drugs, may confer cancer cells resistance to future therapies
The statistical and machine-learning analyses in this work, jointly implemented by Marina Salvadores, a Ph.D. student at the Genome Data Science lab, can connect databases from prior experiments in which many drugs had been tested on cancer cells growing in vitro (in the lab). Moreover, this study also integrated gene editing experimental data, where CRISPR was used to switch off various drug target genes in the same types of cancer cells. This approach allowed the researchers to link drug target genes to drug treatments, thus adding confidence to their main finding that mutational signatures predict drug activity in cancer.
Interestingly, the cancer cells that bear genomic "scars" (mutational signatures) of previous exposure to mutagenic chemicals tended to be resistant to diverse chemotherapeutic drugs. One possible explanation for this is based on the known mechanism by which, for example, brain cancer cells can switch off their DNA repair systems during treatment with the mutagenic drug TMZ, which could permanently covert them into hardy, hypermutating cells resistant to a range of future treatments.
The study suggests that this sort of adaptation may be common in cancer. This has potential implications as tumors caused by mutagen exposure (e.g., lung exposure to tobacco or skin exposure to UV light) may be more difficult to treat, since the cells may harbor a long-term memory of dealing with DNA damage.
The algorithms used to identify the mutational signatures and link them to drug vulnerabilities are open access. Future work by the lab will focus on testing these prediction algorithms on patients' data, thereby overcoming the challenge of the scarcity of public genomic data for patients that correlate with randomized clinical trials.
More information: Jurica Levatić et al, Mutational signatures are markers of drug sensitivity of cancer cells, Nature Communications (2022). DOI: 10.1038/s41467-022-30582-3