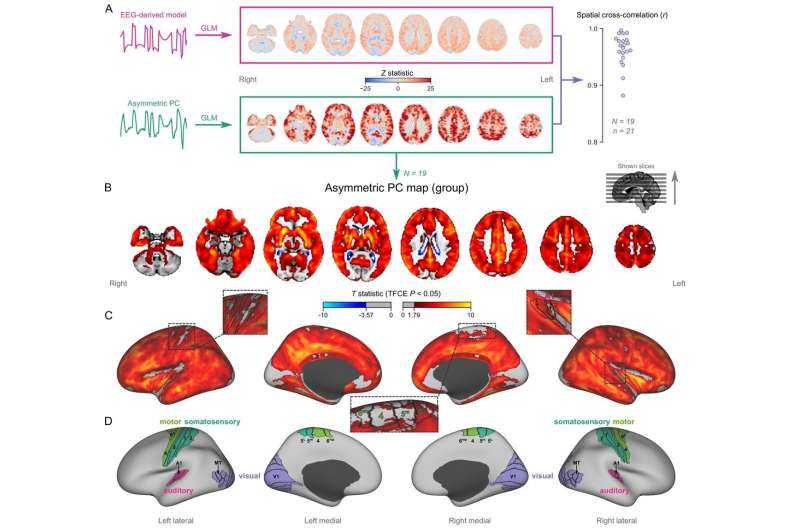
Mapping burst-suppression in anesthetized humans without electroencephalogram (EEG). (A) Maps of burst-suppression are computed via general linear model (GLM) analysis using one of two regressors—either the EEG-derived hemodynamic model or the functional magnetic resonance imaging (fMRI)-derived asymmetric principal component (PC). The resulting Z statistic maps, for an example subject are shown here in the MNI152 template space. Neighborhood cross-correlation values between the two types of Z statistic maps are plotted on the right, across all runs with asymmetric PCs (N=19 subjects, n=21 EEG-fMRI runs, see Figure 2—source data 1). (B) The group burst-suppression map, computed via a second-level analysis of the single-subject asymmetric PC GLMs, is shown here overlaid on the MNI152 volumetric template. The group statistics were carried out with the FSL (FMRIB's Software Library) tool randomize; the resulting T statistic maps were thresholded using threshold-free cluster enhancement (TFCE) and a corrected p<0.05. Figure 2—figure supplement 1 provides a closer look at subcortical structures, while Figure 2—figure supplement 2 examines the source of the observed anticorrelation at the ventricular borders. (C) The group burst-suppression map is shown in fsaverage surface space. Non-cortical areas on the medial surface are shown in dark gray. (D) The locations of several sensory and motor cortical areas, based on the Human Connectome Project multimodal parcellation, are indicated on the surface: primary motor (area 4), premotor (areas 6d and 6mp), primary somatosensory (areas 3a–b, 1, and 2), higher somatosensory (areas 5 m and 5 L), primary auditory, higher auditory (medial and lateral belt, parabelt), primary visual, and higher-order visual (V2, V3, V3A, V3B, V4, V4t, V6, V6A, V7, V8, MT, MST, and lateral occipital areas 1–3). Figure 2—figure supplement 3 shows the unthresholded group burst-suppression map in both volumetric and surface representations. Figure 2—figure supplement 4 shows a temporal signal-to-noise ratio map overlaid on the volumetric template. Credit: eLife (2022). DOI: 10.7554/eLife.74813
Today, anesthesia enables completely painless surgical procedures. It is already known from electroencephalography (EEG) studies on patients that during anesthesia the brain enters a deep sleep-like state in which periods of rhythmic electrical activity alternate with periods of complete inactivity. This state is called burst suppression. Until now, it was unclear where exactly this state happens in the brain, and which brain areas are involved.
However, this question is important to better understand the phenomenon and thus how the brain functions under anesthesia. Researchers from the Functional Imaging Unit at the German Primate Center (DPZ)—Leibniz Institute for Primate Research in Göttingen have used functional magnetic resonance imaging (fMRI) to study the precise spatial distribution of synchronously working brain regions in anesthetized humans, long-tailed macaques, common marmosets and rats. They were able to show for the first time that the areas where burst-suppression is evident differ significantly in primates and rodents. While in rats, large parts of the cerebral cortex synchronously show the burst-suppression pattern, in primates, individual sensory regions, such as the visual cortex, are excluded from it.
"Our brain can be thought of as a full soccer stadium when we are awake," explains Nikoloz Sirmpilatze, a scientist in the Functional Imaging Unit and lead author of the study. "Our active neurons are like tens of thousands of spectators all talking at once. Under anesthesia, however, neuronal activity is synchronized. You can measure this activity using EEG as uniform waves, as if all the spectators in the stadium were singing the same song. In deep anesthesia, this song is repeatedly interrupted by periods of silence. This is called burst suppression. The deeper the anesthesia, the shorter the phases of uniform activity, the bursts, and the longer the periodically recurring inactive phases, the so-called suppressions."
The phenomenon is caused by many anesthetics, which can vary in their mechanisms of action. And burst suppression is also detectable in coma patients. However, it is not known whether this condition is a protective reaction of the brain or a sign of impaired functioning. It has also been unclear where in the brain burst-suppression occurs and which brain areas are involved, as localization by EEG alone is not possible.
To answer this question, Nikoloz Sirmpilatze and the research team used fMRI imaging. The method makes blood flow changes in the brain visible. The increased activity of neurons in a particular area of the brain leads to an increase in metabolism, followed by an increased blood and oxygen supply at this location, which is ultimately visible in the fMRI image.
In the first part of the study, the researchers established a system to evaluate fMRI data in humans, monkeys and rodents in a standardized manner using the same method. To do this, they used simultaneously measured EEG and fMRI data from anesthetized patients that had been generated in a previously conducted study at the Technical University of Munich.
"We first looked to see whether the burst-suppression detected in the EEG was also visible in the fMRI data and whether it showed a certain pattern," says Nikoloz Sirmpilatze. "Based on that, we developed a new algorithm that allowed detecting burst-suppression events in the experimental animals using fMRI, without additional EEG measurement."
The researchers then performed fMRI measurements in anesthetized long-tailed macaques, common marmosets and rats. In all animals, they were able to detect and precisely localize burst suppression as a function of anesthetic concentration. The spatial distribution of burst suppression showed that in both humans and monkey species, certain sensory areas, such as the visual cortex, were excluded. In contrast, in the rats, the entire cerebral cortex was affected by burst suppression.
"At the moment, we can only speculate about the reasons," says Nikoloz Sirmpilatze, who was awarded the German Primate Center's 2021 Ph.D. Thesis Award for his work. "Primates orient themselves mainly through their sense of sight. Therefore, the visual cortex is a highly specialized region that differs from other brain areas by special cell types and structures. In rats, this is not the case. In future studies, we will investigate what exactly happens in these regions during anesthesia to ultimately understand why burst suppression is not detectable there with fMRI."
Susann Boretius, head of the Functional Imaging Unit and senior author of the study, adds: "The study not only raises the question of the extent to which rodents are suitable models for many areas of human brain research, especially when it comes to anesthesia, but the results also have many implications for neuroscience and the evolution of neural networks in general."
More information: Nikoloz Sirmpilatze et al, Spatial signatures of anesthesia-induced burst-suppression differ between primates and rodents, eLife (2022). DOI: 10.7554/eLife.74813
Journal information: eLife
Provided by The German Primate Center