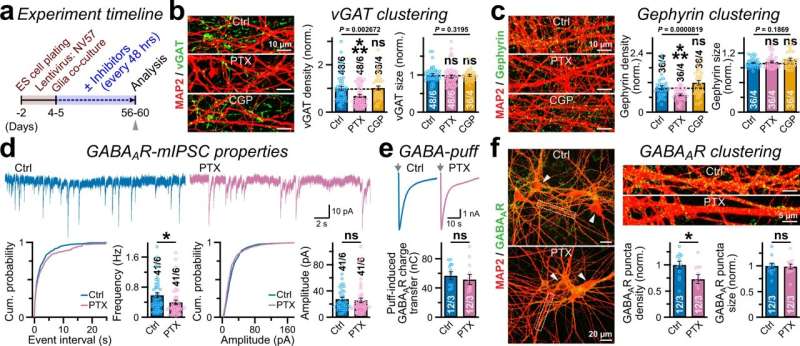
GABAergic synapse properties are modulated by GABAAR activity. a Experimental strategy for panels b–f; NV57 neurons were incubated with synaptic inhibitors, half-exchanged media every other day from post-induction day 4–5 to day 56–60, and analyzed afterwards as indicated (arrow). b, c Sample images (left) and normalized density or size (right) of vGAT b and Gephyrin c clusters formed on MAP2-positive dendrites, when treated with DMSO-only (control), 100 µM PTX, or 10 µM CGP55845. d Representative mIPSC waveforms (top) and event frequency or amplitude (bottom) plotted as cumulative distribution (left) with summary graphs (right), for control vs. PTX-treated (long-term) neurons. Cultures were washed thoroughly with bath-solution before electrophysiological recordings; CNQX was used to stop EPSCs. e Example traces (top) of GABAAR currents produced by 1 mM GABA-puff, and total charge-transfer (bottom). f Sample images (left) of control vs. PTX-treated neurons (arrowheads), as immunostained for extracellular epitopes of surface GABAARs and dendritic MAP2. Boxed regions are magnified (cropped insets, top right), normalized density and sizes of GABAAR clusters are plotted (bottom right), for control vs. PTX-treatment. All summary data are means ± SEM, with total number of cells recorded (electrophysiology) or field-of-views analyzed (imaging) / independent batches, and individual data-points were included as open circles. A near-normal distribution was predicted for most datasets, based on Skewness or Kurtosis values (-2 >≈ and ≈ < 2). Hence, statistical significance was primarily assessed by two-tailed, unpaired, Student’s t-test, with ***P < 0.005; *P < 0.05; ns = not significant, P > 0.05. Multiple groups (b and c) were compared by Kruskal-Wallis test paired with post-hoc nonparametric Mann-Whitney U-test, and corresponding P-values were reported. Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-30756-z
As you're reading this sentence, the cells in your brain, called neurons, are sending rapid-fire electrical signals between each other, transmitting information. They're doing so via tiny, specialized junctions between them called synapses.
There are many different types of synapses that form between neurons, including "excitatory" or "inhibitory," and the exact mechanisms by which these structures are generated remain unclear to scientists. A Colorado State University biochemistry lab has uncovered a major insight into this question by showing that the types of chemicals released from synapses ultimately guide which kinds of synapses form between neurons.
Soham Chanda, assistant professor in the Department of Biochemistry and Molecular Biology, led the study published in Nature Communications that demonstrates the possibility of changing the identity of synapses between neurons, both in vitro and in vivo, through enzymatic means. The other senior scientists who contributed to the project were Thomas Südhof of Stanford University and Matthew Xu-Friedman of the University at Buffalo.
In the lab, Chanda and colleagues were able to make synapses changes between excitatory and inhibitory types, using only enzymes, by making the neurons express just a few genes that induced a cascade of changes in the synapses' machinery. Such a breakthrough could have major implications for treating brain diseases that are caused by malfunctions in synaptic information processing and exchange.
"We know very little about how the human brain functions, and at the center of it, we need to understand how neurons communicate with each other," Chanda said. "Understanding the fundamental mechanisms of synapse formation and maintenance has tremendous implications in understanding brain disorders."
Their results show that the cell-adhesion proteins expressed in the synaptic junction area are not the only purveyors of the synapses' function, as some have thought; rather, chemicals called neurotransmitters that are released from the presynaptic site (where the information is coming from) also seem to play a major role in controlling which types of synapses form, and where.
The CSU team used stem cell-derived human neurons to demonstrate their ability to produce certain types of synaptic connections by controlled release of specific neurotransmitters. Collaborators at the University at Buffalo showed the same phenomenon in live mouse brains.
"Synapses need lots of other machinery; the neurons took care of all that and turned excitatory synapses into inhibitory ones—a fundamental change in their identity," Xu-Friedman said.
Chanda is fascinated by neurons, "because no other cell type in the human body has the same level of functional complexity that is tied so closely to their shape and structure."
More information: Scott R. Burlingham et al, Induction of synapse formation by de novo neurotransmitter synthesis, Nature Communications (2022). DOI: 10.1038/s41467-022-30756-z
Journal information: Nature Communications
Provided by Colorado State University