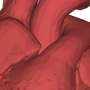
The heart muscle is studded with tiny dyads that coordinate our heartbeats. New research uncovers a vital component that arranges dyads and holds them together. Credit: Medical images: William Pu, MD. Illustrations: Patrick Bibbins, Boston Children’s Hospital
Every heart muscle cell, or cardiomyocyte, is studded with tiny, intricate structures called dyads. The dyads are like orchestra conductors: They coordinate incoming electrical signals with release of calcium in the muscle, triggering contraction. When dyads work properly, the different segments of heart muscle contract in unison; when they don't, heartbeats may be too weak or heart rhythms disrupted.
Dyads are known to be disorganized in many types of heart muscle disease, such as dilated and ischemic cardiomyopathy. But how dyads develop their complex architecture and what leads them to become disorganized has been a mystery. New research led by William Pu, MD, director of Basic and Translational Cardiovascular Research at Boston Children's Hospital, identifies a crucial protein that arranges and holds dyads together.
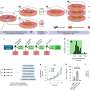
When Pu and colleagues knocked out this protein, called CMYA5 or myospryn, in mouse ventricles, dyad architecture was disrupted. So was calcium release. When they put mechanical stress on the animals' heart muscle, simulating aortic stenosis or chronic hypertension, the muscle couldn't tolerate the overload. The animals' ventricles showed dysfunction on echocardiography and were increased in size.
"We think that CMYA5, or its regulation, is probably abnormal in heart muscle disease," says Pu. "If we could confirm that this protein is disturbed in human heart disease, and show how it's disturbed, we could begin to think of ways to maintain dyad structure and function."
Anatomy of a dyad
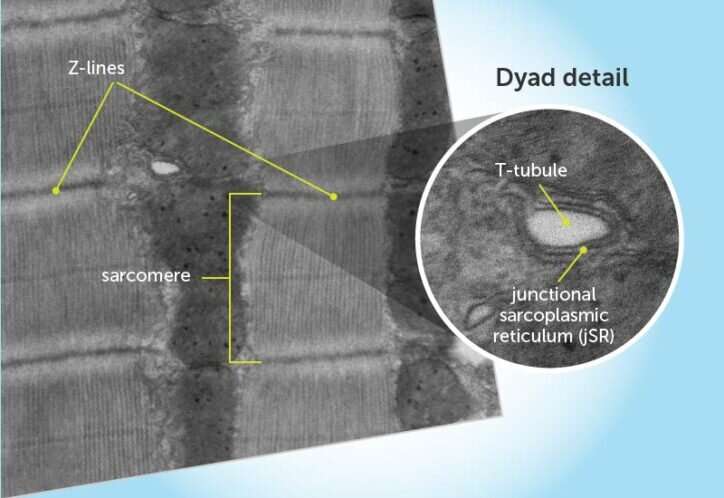
Heart muscle tissue is made of sarcomeres, strings of contractile units that are anchored and separated by Z-lines. Dyads are positioned at the Z-lines at regular intervals. They consist of two main elements: T-tubules (protrusions of the cell membrane into the heart muscle cell that carry the electrical signal) and the junctional sarcoplasmic reticulum (jSR). The jSR releases calcium via ryanodine receptors to initiate contraction.
CMYA5 tethers the jSR to the Z-line, holding the dyad together. Credit: Children's Hospital Boston
To better understand dyads' components, Pu and colleagues studied ventricular cardiomyocytes in live mice, searching for specific proteins that were especially abundant near the dyads. That led them to CMYA5. They went on to show that CMYA5 regulates the stepwise process of dyad assembly and positioning within cardiomyocytes.
To make a dyad, they found, the cell first has to position the jSR next to the Z-line. One side of CMYA5 binds to the Z-line, and the other side binds to the jSR's ryanodine receptor. Only later do the T tubules form, positioned next to the jSR to form a complete dyad.
"CMYA5 is like a tether that holds the Z-line and jSR together," explains Pu. "We think possibly something is going wrong with this tether to contribute to disease. We also showed that the interaction between CMYA5 and the jSR helps regulate the ryanodine receptor, which is important to the functioning and health of heart muscle cells."
Dyads, CMYA5, and heart disease: More to explore
The animal experiments suggest that people with underlying cardiomyopathy or structural heart disease might develop CMYA5 dysfunction, making their heart muscle more likely to fail.
"The dyad is one of many elements in heart muscle that is disrupted in heart disease," says Pu. "We think there are many upstream insults that disrupt dyads and make heart disease worse."
The findings were published in Nature Communications.
More information: Fujian Lu et al, CMYA5 establishes cardiac dyad architecture and positioning, Nature Communications (2022). DOI: 10.1038/s41467-022-29902-4
Journal information: Nature Communications
Provided by Children's Hospital Boston