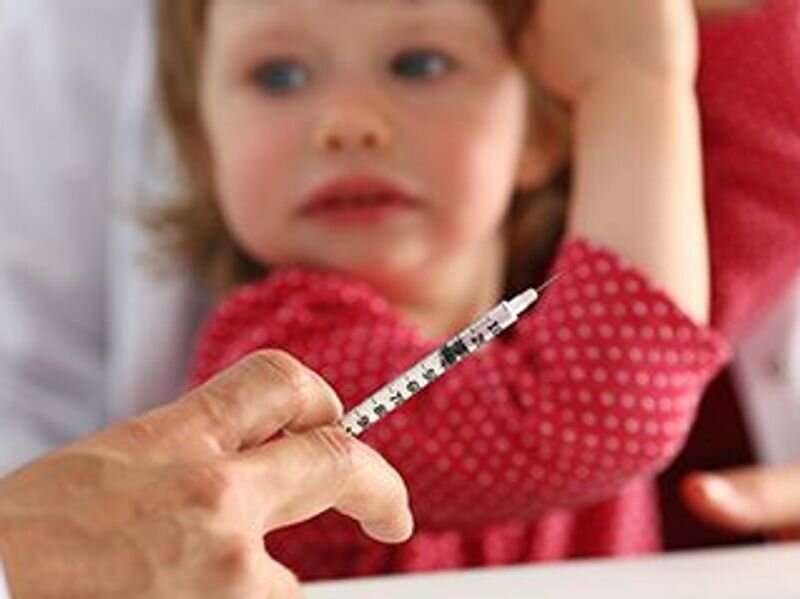
Pfizer Inc. said Wednesday that it has asked the U.S. Food and Drug Administration to approve the emergency use of its COVID-19 vaccine in children younger than the age of 5 years.
The company said in a statement that it has provided the agency with data from a phase 2/3 trial that included almost 1,700 children who received a third dose of the vaccine when omicron was the dominant variant.
The trial showed that the vaccine triggers a strong immune response and is safe. A month after the third dose, antibody levels in the children were similar to those seen in 16- to 25-year-olds after two doses, according to the company. At midtrial, the vaccine was 80.3 percent effective in preventing symptomatic COVID-19. The findings were released May 23, but they have not yet been peer-reviewed or published in a medical journal.
The children in the trial received three 3-mcg doses, with the first two doses given three weeks apart= and the third dose given at least two months after the second dose. The doses for children ages 6 months to 5 years are smaller than those in older groups. Children ages 5 to 12 years receive two doses of a 10-mcg vaccine, and people 12 years and older are given two doses of a 30-mcg vaccine. Both groups are eligible for booster doses, CNN reported.
Children younger than 5 years are the only age group in the United States not eligible for COVID-19 vaccination. In late April, Moderna provided the FDA with trial data on the use of its COVID-19 vaccine in children ages 6 months to 5 years. An FDA panel of vaccine experts is set to meet on June 15 to discuss both the Moderna and Pfizer requests for the emergency use of COVID-19 vaccines among these younger children.
More information: CNN Article
© 2022 HealthDay. All rights reserved.