Tumors with specific genetic mutations show response to immune checkpoint blockade therapy
New research from a collaborative team at Cleveland Clinic, led by Timothy Chan, MD, Ph.D., Chair of the Center for Immunotherapy and Precision Immuno-Oncology in the Lerner Research Institute, is demonstrating that pathogenic Polymerase epsilon and delta (POLE/POLD1) genetic mutations lead to improved response to immune checkpoint blockade (ICB), a growing class of immunotherapy drugs.
The findings, published in Nature Genetics, contribute to a growing list of discoveries that prove certain classes of drugs are more effective in tumor-agnostic cases where the genetic makeup of the tumor, and not the histology of the tumor, should determine the decision-making process for treatment.
"To date, this is the most comprehensive analysis of the mutations in POLE/POLD1 genes in patients with cancer," says Dr. Chan. "The basis of this research is built upon our previous knowledge showing that the more mutations a tumor has, the better the response to ICB therapy. Our research here shows that patients with POLE/POLD1 mutations should get immune checkpoint blockade therapy as a preferred treatment, and that POLE/POLD1 mutations are a potential tumor-type agnostic indication for immunotherapy."
POLE/POLD1 are DNA damage response and repair (DDR) genes that are genetically altered in nearly 4% of tumors across all cancer types. In these cases, about 10–13% are due to germline variants—a genetic change in the DNA of every offspring cell. However, not all the POLE/POLD1 mutations are pathogenic or functional that could lead to sensitivity to immunotherapy.
Tumors containing pathogenic POLE/POLD1 mutations have high levels of immune cell infiltrations, and until this point, it was challenging to distinguish these pathogenic mutations from non-functional ones. It remained unclear how these mutations would affect patients' response to anti-tumor immunity therapy.
In preclinical models, the growth of tumors introduced with well-established POLE/POLD1 pathogenic mutations was dramatically inhibited by either mono- or combination ICB therapies. In contrast, treatment had little effect on growth of the parental POLE/POLD1 wild-type tumors.
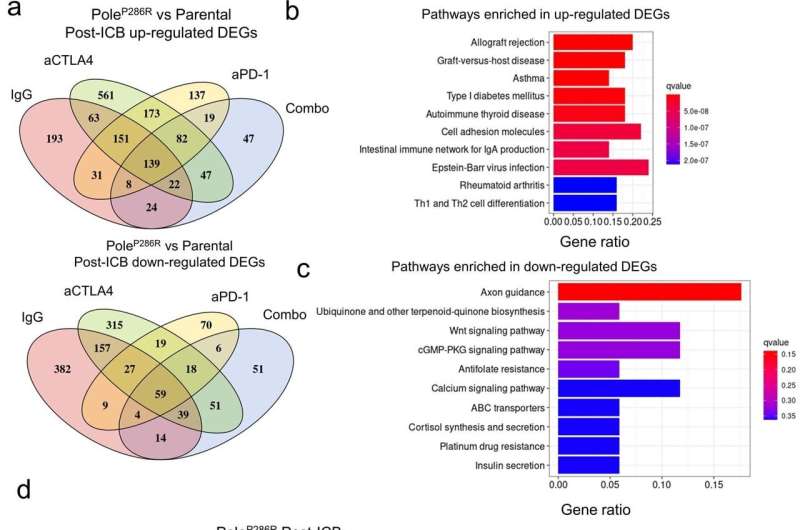
To determine whether similar phenomena could be observed in patients, the team generated statistical learning models based on mutational signatures—such as the imprints of DNA damage and repair process on genome occurred during tumorigenesis—of the POLE/POLD1 mutant tumors. These signatures could identify the telltale DNA fingerprints of functional POLE/POLD1 mutations. Modeling demonstrated high accuracy for distinguishing the tumors with pathogenic POLE/POLD1 mutations from those tumors. The researchers further applied one of these models to a retrospective pan-cancer immunotherapy cohort curated at Memorial Sloan Kettering Cancer Center and found that their model outperformed other traditional approaches for identifying the patients with pathogenic POLE/POLD1 mutations that are more likely to benefit from immunotherapy.
Further analysis indicated that the underlying mechanism is associated with the increased quantity and quality of the immunogenic mutations, as well as the improved immune microenvironment in the tumors harboring pathogenic POLE/POLD1 mutations.
"The short-term impact of this study on cancer immunotherapy is that we comprehensively evaluated and demonstrated that tumors with functional POLE/POLD1 mutations are sensitive to immunotherapy, and patients with this type of tumor could benefit more from immunotherapy," says Xiaoxiao Ma, Ph.D., a postdoctoral researcher at Cleveland Clinic and lead author of the study. "The long-term impact is that we demonstrate it's possible to utilize genomic features, such as mutational signatures, to identify a major indication for immune checkpoint therapy."
"Providing efficient treatment strategies in a precise way for people with cancer is one of the long-term research goals of our lab," says Dr. Ma. "This, we know, is the key to future cancer treatment."
More information: Xiaoxiao Ma et al, Functional landscapes of POLE and POLD1 mutations in checkpoint blockade-dependent antitumor immunity, Nature Genetics (2022). DOI: 10.1038/s41588-022-01108-w