Carbon oxide gas boosts photothermal therapy under mild temperature
Photothermal therapy (PTT) is a safe cancer hyperthermia strategy that utilizes photothermal conversion agents to convert light energy into heat to ablate cancer cells.
On one hand, high-temperature (>50 ℃) PTT causes an unavoidable threat to surrounding healthy tissues and could induce inflammatory disease because of the difficulty in blocking heat diffusion. On the other hand, the ablation effect at relative low-temperature (<45 ℃) is far from sufficient due to the upregulated expression of the heat shock proteins (HSPs), which repair the thermally damaged cells and lead to the thermotolerance of tumor cells upon laser irradiation. It's imperative to develop new strategy for HSPs silencing for low temperature PTT.
Now, a research team led by Dr. Cai Lintao from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences has reported a new strategy using carbon oxide gas to inhibit the expression of HSPs, providing an alternative strategy for boosting low-temperature PTT.
The study was published in Angewandte Chemie International Edition on July 15.
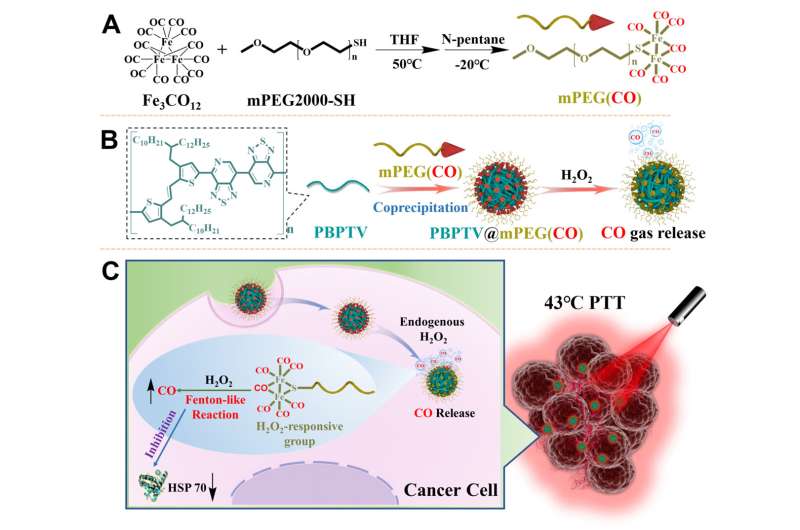
In the experiment, the researchers developed a chemiexcitation-triggered photo-active nanodelivery system (termed AIE nanobomb) based on the self-assembly of NIR-II luminescent polymer with aggregation-induced emission properties (PBPTV) and home-made carbon monoxide (CO) carrier polymer mPEG(CO). The nanobomb could triggered by the high level of H2O2 in tumor microenvironment and selectively release CO gas in tumor cells.
In the cancerous microenvironment, over-secreted H2O2 diffuses across the nanobomb to preferentially decompose into ·OH radicals under the catalysis of FeCO via a Fenton-like reaction, and strongly oxidative ·OH radicals further oxidize and competitively coordinate with the Fe center, causing the release of CO from the Fe center. The gradually released CO could effectively inhibit the elevated expression of the HSPs to destroy tumor thermal resistance during the low-temperature PTT process and induced the tumor apoptosis.
"As a safe cancer treatment modal, photothermal therapy under mild temperature could not only induced the tumor apoptosis, but also activate the immune system to attack the residue tumor cell in human body and eliminate the tumor recurrence," said Dr. Cai. "We call this treatment photo-immuno-therapy. There is no doubt that how to selectively inhibit expression of the heat shock proteins in tumor cells and reverse thermotolerance of the cell is the key."
Small molecular HSPs inhibitor and small-interfering RNA (siRNA) have been widely co-loaded with photothermal conversion agents to improve the therapeutic effect of low temperature PTT. Small molecular HSPs inhibitor are either antibiotics or anticancer agents, such as tanespimycin (also known as 17-AAG), gambogic acid, and so on.
"They are almost hard to be dissolved in water and have side effect to normal cells, and siRNA seems to be a good approach. However, they are so easy to be degraded in human body," said Dr. Zhang Pengfei, the main contributor of this study, "we are also surprised that carbon oxide works. It inspires us that nothing is impossible."
"As a signaling molecule, carbon oxide (CO) can trigger a series of cellular protective mechanisms in stress and inflammation. The mechanism of CO down regulating HSPs protein is still unclear," said Dr. Gong Ping Gong, another main contributor of this study, "according to some literature, we may hypothesize that it might be related with LKB1/AMPK/mTOR pathway, but a large amount work is still needed to be done to prove it."
PBPTV is an ambipolar pyridal thiadiazole-based semiconducting polymer, the use of bis-pyridal-thiadiazole unit enables PBPTV to achieve high electron affinity, low LUMO level, and extended π-conjugation, which has shown great potential for designing high-performance electron-transporting semiconductors in organic electronics.
"We had never thought it could emit light before, and never thought it could be used in biomedicine. This work gives us an idea of the promise of our material. I think it's important to work across disciplines and I will continue to work with the research team at SIAT," said Dr. Huajie Chen from Xiangtan University, a collaborator in this work.
"Gas therapy is an emerging and promising field, although there are some reports on combination of gas therapy with photo therapy for cancer. The interaction between gas and biological process might open new door to solve some existing problem in disease therapy. Besides, there are also clinical reports based on gas therapy. Using gas to solve the problem in photo therapy is also a good example of drug repurposing," said Dr. CAI.
More information: Gongcheng Ma et al, H 2 O 2 ‐Responsive NIR‐II AIE Nanobomb for Carbon Monoxide Boosting Low‐Temperature Photothermal Therapy, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202207213