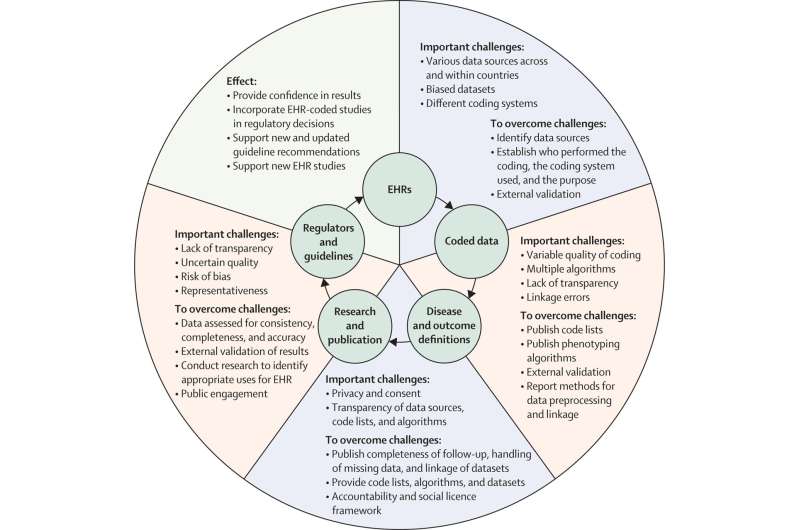
From structured health-care data to improved patient care Key challenges and the paths to improvement leading to sustainable impact from EHR-based research studies. EHR=electronic health-care record. Credit: The Lancet Digital Health (2022). DOI: 10.1016/S2589-7500(22)00151-0
Routinely-collected health care data has the potential to improve the lives and well-being of patients across the world, through better understanding of disease, and research on existing and new treatments. Presented today at the ESC Congress and simultaneously published in The BMJ, The Lancet Digital Health and European Heart Journal, an international team propose a framework to improve the integrity and quality of studies using health care data, and boost confidence in using the results for clinical decision support.
The approach was compiled by a wide range of global stakeholders, coordinated by the BigData@Heart consortium and the European Society of Cardiology. This included patients and patient advocacy groups, regulators, government agencies and leading medical journals, plus representatives from professional societies, academic institutions, the pharmaceutical industry and payers. Participants convened to review opportunities and challenges, and develop pragmatic advice on how health care data can be applied to research across the spectrum of disease.
"Health data science has undergone rapid development in recent years, including the common adoption of electronic health care record systems. However, concerns over quality, data privacy, transparency and comparability have limited the use of evidence generated using structured health care data. These issues have also restricted acceptance by regulators, reimbursement authorities and guideline task forces," states the paper.
Author Professor Dipak Kotecha of the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, U.K. said, "With the support of patients and the public, routinely collected health care information provides an exciting opportunity to answer important clinical questions in populations representative of our communities. Our ability to apply findings from studies that use these data sources is critically dependent on transparency at every stage. This international framework will enable robust and effective use of health care data for clinical research and provide those working in this field with guidance on how to design better studies for maximal benefit to patient care."
The CODE-EHR framework was iteratively developed to provide researchers with step-by-step guidance on how to achieve appropriate governance and transparency, and for stakeholders to have confidence in the reported findings. Minimum standards are outlined for five key areas, with preferred standards providing the direction for future research:
- Dataset construction and linkage; clarifying the source, completeness and linkage of any health care data used in the study.
- Data fit for purpose; providing detail on the coding systems used, any data manipulation, and assessment of data quality.
- Disease outcome and definitions; allowing other researchers to re-use and improve by clearly stating all codes and algorithms used, including those for patient identification, therapy, procedures, comorbidities and outcomes.
- Analysis; describing how outcome events were analyzed to allow for validation and replication.
- Ethics and governance; communicating processes for consent, data privacy, and patient and public involvement.
Report author Professor Folkert Asselbergs of the University Medical Center Utrecht, the Netherlands and University College London, U.K. said, "The use of real-world data in large-scale registries and randomized trials is ushering in a new era of clinical evidence generation. The CODE-EHR framework addresses public concerns about data sharing and provides greater clarity on the use of real-world health care data for a broad range of stakeholders to improve clinical care."
More information: Dipak Kotecha et al, CODE-EHR best practice framework for the use of structured electronic healthcare records in clinical research, BMJ (2022). DOI: 10.1136/bmj-2021-069048
Dipak Kotecha et al, CODE-EHR best-practice framework for the use of structured electronic health-care records in clinical research, The Lancet Digital Health (2022). DOI: 10.1016/S2589-7500(22)00151-0
Dipak Kotecha, CODE-EHR best practice framework for the use of structured electronic healthcare records in clinical research, European Heart Journal (2022). DOI: 10.1093/eurheartj/ehac426
Journal information: European Heart Journal , British Medical Journal (BMJ)
Provided by European Society of Cardiology