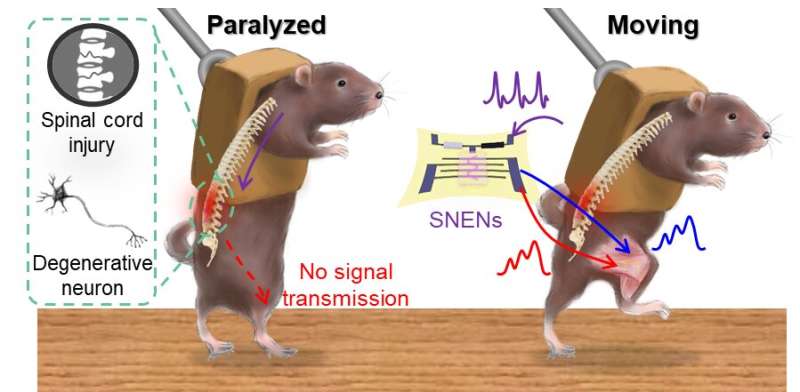
Mice with impaired spinal cord or motor nerve (left), recovery of motor function in mice with impaired nerve through stretchable artificial nerves (right). Credit: Seoul National University
An international research team led by Prof. Tae-Woo Lee (Department of Materials Science and Engineering, Seoul National University, Republic of Korea) and Prof. Zhenan Bao (Department of Chemical Engineering, Stanford University, US) has succeeded in recovering muscle movements in a model of paralyzed mice through organic artificial nerves. The result was published in Nature Biomedical Engineering.
The nerves, which are essential for life activities as well as having a significant impact on quality of life, are easily damaged by various causes such as physical injury, genetic causes, secondary complications, and aging. In addition, once nerves are damaged they are difficult to reconstruct, and some or all their bodily functions are permanently lost due to poor bio-signaling.
Among the various methods for rehabilitation in patients with neurological damage, Functional Electrical Stimulation (FES), which is currently actively used in clinical practice, uses computer-controlled signals. Through this setup, electrical stimulation is applied to muscles that are no longer arbitrarily controllable in patients with neuropathy to induce muscle contraction, resulting in functionally useful movements in the biological body even though they are confined in a specific space. However, this conventional approach has limitations that are not suitable for long-term use in patients' daily lives because they involve complex digital circuits and computers for signal processing to stimulate muscles, consuming a lot of energy and poor biocompatibility in the process.
To solve the problem, the research team succeeded in controlling the leg movement of mice only with artificial nerves without a complex and bulky external computer using stretchable, low-power organic nanowire neurormorphic devices that emulate the structure and function of bio nerve fibers. The stretchable artificial nerve consists of a strain sensor that simulates a proprioceptor which detects muscle movements, an organic artificial synapse that simulates a biological synapse, and a hydrogel electrode for transmitting signals to the leg muscles.
The researchers adjusted the movement of the mouse legs and the contraction force of the muscles according to the firing frequency of the action potential transmitted to the artificial synapse with a principle similar to that of the biological nerve, and the artificial synapse implements smoother and more natural leg movements than the usual FES.
In addition, the artificial proprioceptor detects the leg movement of the mouse and gives real-time feedback to the artificial synapse to prevent muscle damage due to excessive leg movement.
The researchers succeeded in allowing a paralyzed mouse to kick the ball or walk and run on the treadmill. Furthermore, the research team showed the applicability of artificial nerves in the future for voluntary movement by sampling pre-recorded signals from the motor cortexes of moving animals and moved the legs of mice through artificial synapses.
The researchers discovered a new application feasibility in the field of neuromorphic technology, which is attracting attention as a next-generation computing device by emulating the behavior of a biological neural network.
Prof. Tae-Woo Lee says that "neural damage is still considered a big scientific challenge from the past to the present despite the remarkable advances in medicine, and without a new breakthrough, it will still remain a hard-to-solve problem in the future. This research provides a new breakthrough in overcoming nerve damage in an engineering way using neuromorphic technology, not in a biomedical way," expressing the significance of the study. He also added, "An engineering approach to overcoming nerve damage will open a new path to improving the quality of life for those suffering from related diseases and disorders."
Prof. Zhenan Bao, noted the potential of the study, saying that "through the development of stretchable artificial nerves for nerve-damaged patients, it has provided a cornerstone for patient-friendly, more practically usable wearable neural prosthetics, away from the existing form factor."
More information: Zhenan Bao, A low-power stretchable neuromorphic nerve with proprioceptive feedback, Nature Biomedical Engineering (2022). DOI: 10.1038/s41551-022-00918-x. www.nature.com/articles/s41551-022-00918-x
Journal information: Nature Biomedical Engineering
Provided by Seoul National University