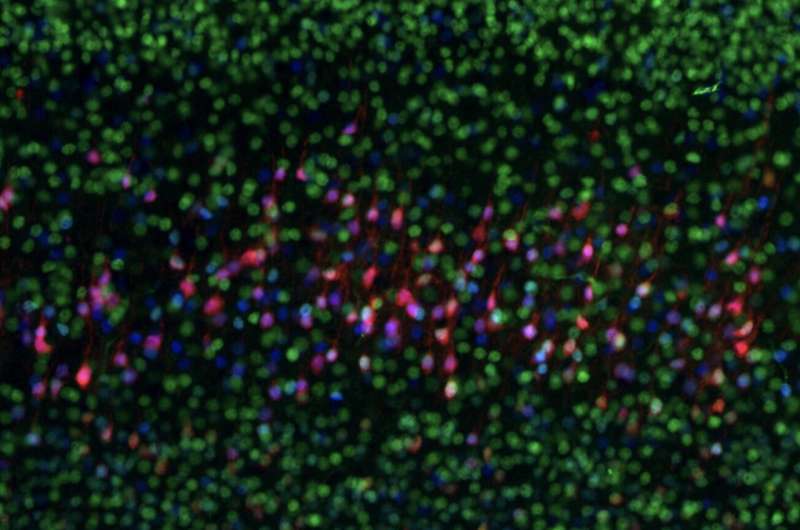
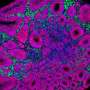
A close-up of the layers of the mouse cortex, showing excitatory neurons characteristic of different layers, which connect the cortex to different brain areas. Callosal projection neurons in the upper layers, which primarily connect to other parts of the cortex, have the highest expression of SATB2 (green), while subcerebral projection neurons expressing FEZF2 (red) and CTIP2 (blue), which connect predominantly to brain areas outside the cortex, are most common in Layer 5, towards the middle of the cortex. Credit: Daniela Di Bella.
Neurons in the cerebral cortex, a region in the mammalian brain involved in complex motor functions, sensory perception and cognition, can have very different qualities and characteristics. These neurons are known to be formed during embryonic development, and their properties and functions keep evolving after birth.
Many past neuroscience studies have investigated the formation and development of cortical neurons. However, so far none of these have been able to clearly define the regulatory mechanisms that underpin their progressive maturation before and after birth.
Researchers at Harvard University, Broad Institute, Massachusetts Institute of Technology (MIT), the Max Planck Institute of Psychiatry and other institutes in Europe have recently carried out an in-depth integrated single-cell epigenomic and transcriptional analysis of individual cortical cells in the brain of mice and marmosets. Their findings, published in Nature Neuroscience, shed some new light on the regulatory strategies that control the early and late development of different types of cells in the neocortex.
"We wanted to understand how neurons in the cerebral cortex, the region of the brain responsible for skilled motor function, sensory perception, and cognition, are generated and wired together," Paola Arlotta, one of the researchers who carried out the study, told MedicalXpress. "We have been studying these processes for almost two decades and our recent study adds another piece to the puzzle."
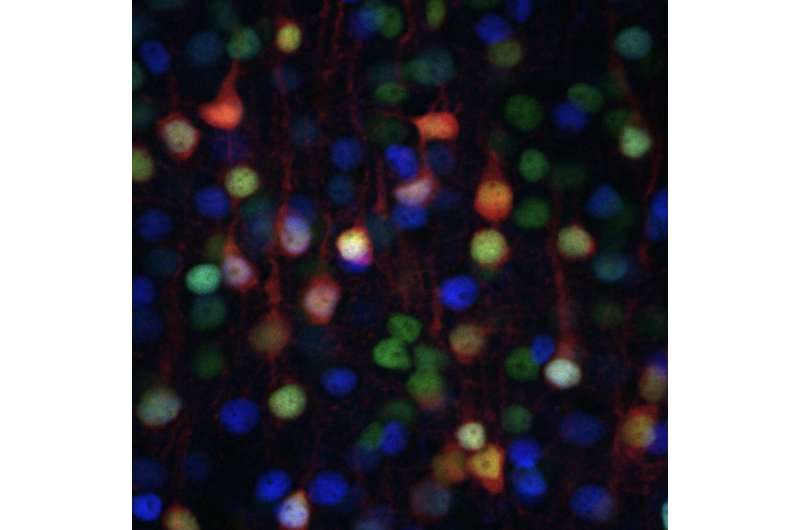
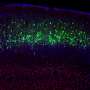
A close-up of Layer 5 of the mouse cortex, showing different types of excitatory neurons, which connect the cortex to different brain areas. Different neuron types in Layer 5 express combinations of SATB2 (green), FEZF2 (red), and CTIP2 (blue). Credit: Daniela Di Bella.
To gain more insight about the regulatory strategies underpinning the development of cortical neurons, Arlotta and her colleagues carried out several analyses using different state-of-the-art technologies and genetic methods. These methods allowed them to define the genes that were preferentially expressed in individual types of neurons at different ages.
The team analyzed cortical neurons from the brains of mice and marmosets of different ages. At these different ages, the neurons are at different stages of development, thus they have different characteristics.
"We looked at multiple ages, when neurons are doing different things, such as being born or making connections to other neurons or responding to environmental stimuli," Arlotta explained. "We also looked at whether regions of the genomes that regulate the production of such genes were available at an age or another. This allowed us to define whether genes were active and what mechanisms were in place to control them. We looked over time but also across species."
-
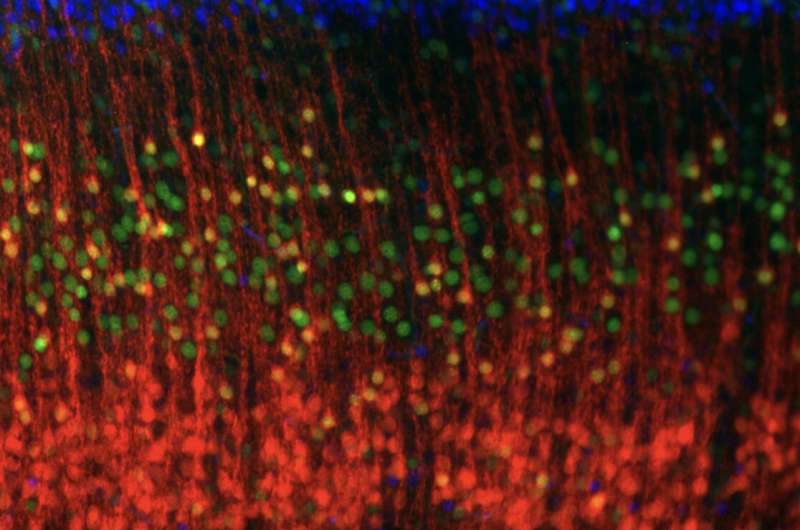
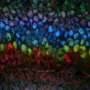
A close-up of the layers of the mouse cortex, showing excitatory neurons characteristic of different layers, which connect the cortex to different brain areas: callosal projection neurons in the upper layers express CUX1 (blue) and predominantly connect to other areas of the cortex; subcerebral projection neurons expressing CTIP2 (green), which are most common in Layer 5, towards the middle of the cortex, connect predominantly to brain areas outside the cortex; and corticothalamic projection neurons expressing TLE4 (red), which are most common in the deep layers, connect predominantly to the thalamus. Credit: Daniela Di Bella.
-
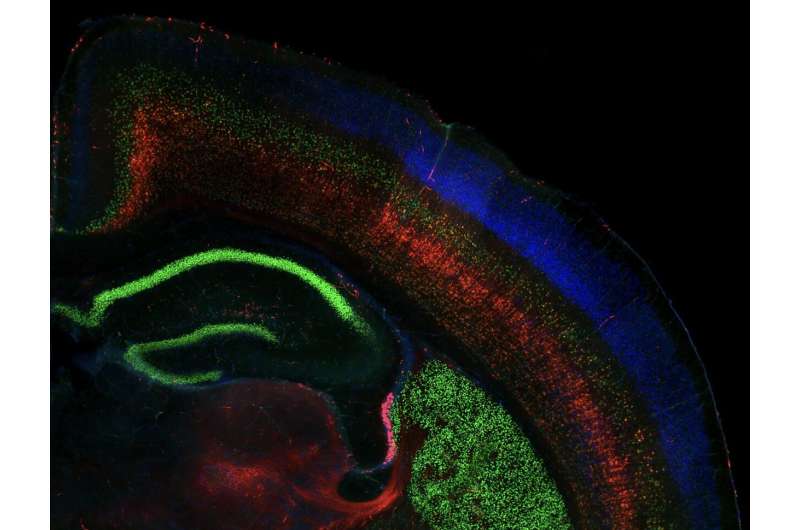
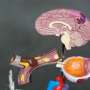
A section of the mouse brain, showing a portion of one hemisphere of the cortex overlying the hippocampus (bottom left) and striatum (bottom right). Excitatory neurons characteristic of different cortical layers connect the cortex to different brain areas: callosal projection neurons in the upper layers express CUX1 (blue) and predominantly connect to other areas of the cortex; subcerebral projection neurons expressing CTIP2 (green), which are most common in Layer 5, towards the middle of the cortex, connect predominantly to brain areas outside the cortex; and corticothalamic projection neurons expressing TLE4 (red), which are most common in the deep layers, connect predominantly to the thalamus. Credit: Daniela Di Bella.
The single-cell epigenomic and transcriptional analysis conducted by the researchers yielded several interesting findings. Most notably, Arlotta and her colleagues found that early in the life of neurons, around the time when they are born, the cortex regulates their genes using "simpler" regulation strategies.
"It turns out that such regulatory mechanisms are more broadly used by other cell types in the body, even outside the brain, and are conserved during evolution; like a screenplay that has been used before," Arlotta said. "However, later, when neurons wire and respond to environmental cues, they use more 'sophisticated' mechanisms of regulation that appear to be more unique to the brain; almost like a new screen play that needs to depict some more dynamic aspects of the biology of the neurons."
The recent work by this team of researchers showed that as cortical neurons develop and mature, the cerebral cortex in the mice and marmoset brain adapts the strategies with which it regulates their genes. In their paper, Arlotta and her colleagues suggests that this temporal shift in regulatory mechanisms could reflect unique evolutionary constraints on different developmental events occurring in the neocortex.
"I like to think about our findings as follows: the evolutionary variability in the mechanisms that control the making of brain cells may be somewhat contained, most likely because mistakes in this process would not be easily tolerated," Arlotta added. "However, when neurons need to wire and dynamically adapt to the environment, then it is advantageous for them to have more degrees of freedom in gene regulation space to allow for more complex and perhaps unpredictable, functional outcomes."
More information: Wen Yuan et al, Temporally divergent regulatory mechanisms govern neuronal diversification and maturation in the mouse and marmoset neocortex, Nature Neuroscience (2022). DOI: 10.1038/s41593-022-01123-4
Journal information: Nature Neuroscience
© 2022 Science X Network