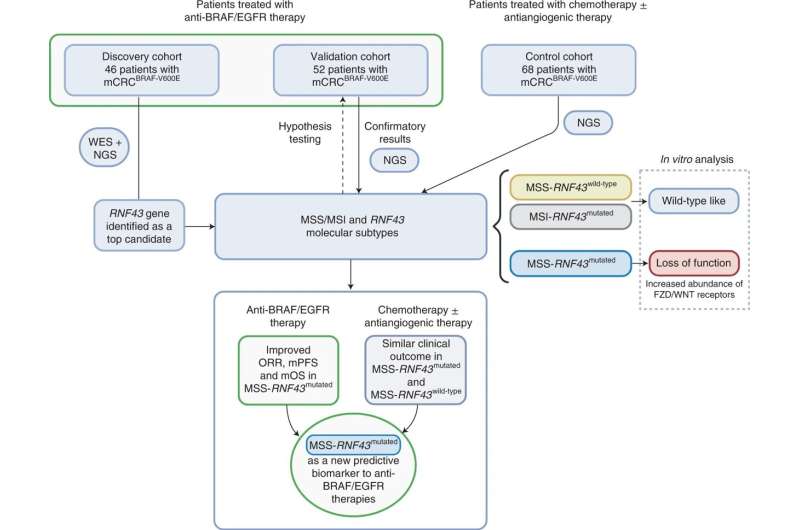
A total of 166 patients with mCRCBRAF-V600E were included in the study from discovery (n = 46), validation (n = 52) and control (n = 68) cohorts. WES of germline DNA, baseline tumor DNA and/or baseline plasma cfDNA from 28 patients was performed. Targeted NGS was used to assess RNF43 tumor mutation status for the 18 remaining patients from the discovery cohort and all tumors from the validation and control cohorts. Genomic profiles and MSS/MSI-RNF43 molecular subtypes were compared with clinical response data (ORR, mPFS and mOS) using dNdScv maximum-likelihood unbiased mutation enrichment analysis. In vitro assays were used to assess the functional impact of RNF43 mutations detected in patient samples. Credit: Nature Medicine (2022). DOI: 10.1038/s41591-022-01976-z
Results of a study led by VHIO investigators and published in Nature Medicine have unmasked mutations in the RNF43 gene as predictive biomarkers of response to treatment with anti-BRAF/EGFR combinatory therapy in patients with microsatellite stable BRAF V600E metastatic colorectal cancer (MSS mCRC). Data show that patients with tumors harboring loss-of-function mutations in RNF43 respond favorably to dual BRAF/EGFR blockade and achieve improved progression-free as well as overall survival rates.
Mutations in BRAFV600E occur in around 10% of mCRC, and while relatively rare, they associate with a poor prognosis. Patients with tumors harboring this alteration are generally refractory to treatment and rapidly develop cancer drug resistance. In 2019, findings from the phase III BEACON trial, co-led by VHIO's Director Josep Tabernero, rang in a new standard of care with the doublet combination of BRAF (encorafenib) and EGFR (cetuximab) inhibitors in patients with previously treated BRAF V600E-mutated mCRC.
"While this combinatory therapy can significantly improve outcomes in some of these patients, others do not show benefit. Up until now, the underlying genetic determinants of efficacy have not been described. We therefore sought to determine which genes were enriched for somatic mutations in responder and non-responder groups," says Elena Élez, a Senior Clinical Investigator of VHIO's Gastrointestinal and Endocrine Tumors Group, and a corresponding author of this present study alongside Rodrigo A. Toledo, a Translational Investigator of the same group.
46 patients from the Vall d'Hebron University Hospital—HUVH (Vall d'Hebron Barcelona Hospital Campus), were prospectively included in the discovery cohort, and 52 patients from the other three participating university hospitals in Italy were included in the validation group. To confirm the study findings from both cohorts treated with anti-BRAF/EGFR, the investigators assessed data from 68 additional patients who had received other treatments such as chemotherapy plus antiangiogenic agents -without anti-BRAF therapy- at the four participating hospitals.
"We performed extensive genomic analysis of over 20,000 genes. In total, we analyzed data from 166 patients which represents a significant number considering the rarity of this tumor subtype that accounts for around ten percent of all colorectal cancers," says Rodrigo A. Toledo, co-corresponding author of this study and a Translational Investigator at VHIO.
The genomic analysis of responders versus non-responders showed that RNF43 mutations, identified in 29% of BRAFV600E MSS mCRC "cold" tumors, strongly associated with clinical response to anti-BRAF/EGFR-based combinations in these patients. Results show that those patients presenting these mutations responded better than those who did not, with a better response rate, longer progression-free as well as overall survival.
The response rate among patients with a BRAFV600E MSS mCRC carrying a RNF43 mutation reached 72.7%, and only 30.8% in those without. In patients with tumors harboring the RNF43 mutation, median disease-free progression was 10.1 months versus 4.1 months. Importantly, overall survival in the former was 13.6 months compared to 7 months in the latter.
"Our data point to RNF43 as a potential stratification biomarker that could help steer treatment decision making as well as define the optimal sequence of treatment in patients with microsatellite stable, BRAF V600E- mutant metastatic colorectal cancer. As importantly, it could also help to identify those patients for whom alternative treatment options are very much needed," adds Toledo.
While these results could usher in a much-needed biomarker of response in this setting, the study authors suggest that further research should seek to incorporate this candidate biomarker in routine testing along with BRAF and MSS/Microsatellite Instability (MSI) status and evaluate their possible integration with other transcriptomic, microbiome, and microenvironmental indicators.
"The discovery and validation of robust biomarkers of drug response in this patient population will help to prioritize anti-EGFR/BRAF combinations in those patients who are more likely to derive benefit, as well as optimize the clinical management of such a heterogeneous and highly complex disease," concludes Elena Élez, a Medical Oncologist at Vall d'Hebron's Medical Oncology Department headed by Josep Tabernero, also a co-author of this present study.
More information: Elena Elez et al, RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAFV600E metastatic colorectal cancer, Nature Medicine (2022). DOI: 10.1038/s41591-022-01976-z
Journal information: Nature Medicine
Provided by Vall d'Hebron Institute of Oncology