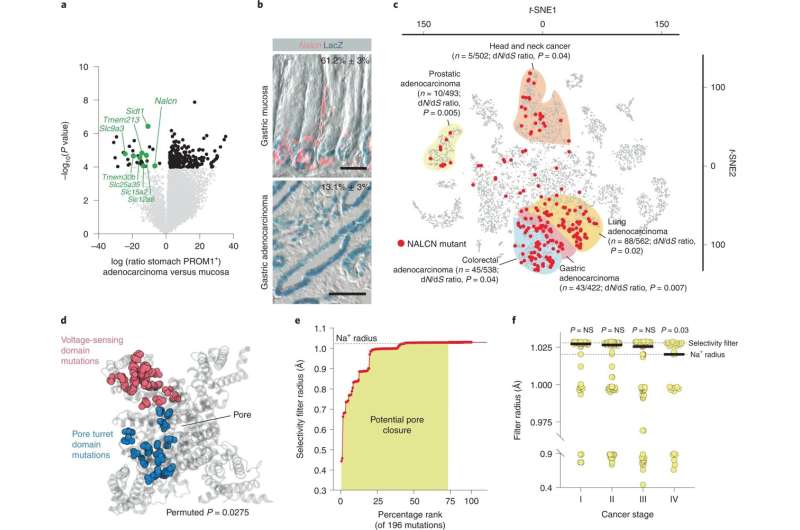
NALCN loss-of-function in aggressive cancers.a, Differential gene expression between normal PROM1+ gastric cells and P1KP-GAC cells (downregulated ion channels are highlighted). Benjamini–Hochberg corrected P value, alpha = 0.05. b, Nalcn RNA in situ hybridization and Prom1 expression (β-galactosidase (LacZ)) in Prom1CreERT2/LacZ mouse stomach (n = 3 biological replicates, 10 fields each; upper) and P1KP-GAC (n = 3 biological replicates, 10 fields each; lower). Numbers are shown as mean ± s.e.m. Prom1+/Nalcn+ cells. Scale bar, 50 μm. c, t-SNE plot of 10,022 human cancers (P value, dN/dS shown; Source data). d, Mutant residues enriched in NALCN pore turret (blue) and voltage-sensing (red) domains. P = 0.0275; permuted P value for probability of observing two clusters of 20 and 25 residues. e, Impact of 196 NALCN mutations on selectivity filter radius determined by HOLE analysis. f, NALCN pore closure by NALCN mutations in stage I (n = 47), stage II (n = 73), stage III (n = 74) and stage IV (n = 27) human cancers. Two-tailed Mann–Whitney U-test: stage I versus II, P = 0.3488; stage I versus III, P = 0.1613; stage I versus IV, P = 0.0293. Bar denotes median filter radius. Credit: Nature Genetics (2022). DOI: 10.1038/s41588-022-01182-0
Scientists have discovered that cancer cells "hijack" a process used by healthy cells to spread around the body, completely changing current ways of thinking around cancer metastasis.
The team based at the Cancer Research UK Cambridge Institute, University of Cambridge, found that blocking the activity of the NALCN protein in cells in mice with cancer triggers metastasis.
The research, which was published in Nature Genetics, also discovered that this process is not just restricted to cancer. To their surprise, when they removed NALCN from mice without cancer, this caused their healthy cells to leave their original tissue and travel around the body where they joined other organs.
They found, for example, that healthy cells from the pancreas migrated to the kidney where they became healthy kidney cells. This suggests that metastasis isn't an abnormal process limited to cancer as previously thought, but is a normal process used by healthy cells that has been exploited by cancers to migrate to other parts of the body to generate metastases.
Group Leader for the study and Director of the Cancer Research UK Cambridge Centre, Professor Richard Gilbertson, says that "these findings are among the most important to have come out of my lab for three decades. Not only have we identified one of the elusive drivers of metastasis, but we have also turned a commonly held understanding of this on its head, showing how cancer hijacks processes in healthy cells for its own gains. If validated through further research, this could have far-reaching implications for how we prevent cancer from spreading and allow us to manipulate this process to repair damaged organs."
Despite being one of the main causes of death in cancer patients, metastasis has remained incredibly difficult to prevent, largely because researchers have found it hard to identify key drivers of this process which could be targeted by drugs. Now that they have identified NALCN's role in metastasis, the team are looking into various ways to restore its function, including using existing drugs on the market.
Lead researcher on the study and Senior Research Associate at the Cancer Research UK Cambridge Institute, Dr. Eric Rahrmann, says that they "are incredibly excited to have identified a single protein that regulates not only how cancer spreads through the body, independent of tumor growth, but also normal tissue cell shedding and repair. We are developing a clearer picture on the processes that govern how cancer cells spread. We can now consider whether there are likely existing drugs which could be repurposed to prevent this mechanism from triggering cancer spreading in patients."
Cancer Research UK's Director of Research, Dr. Catherine Elliott, says that "once cancer has spread from the first tumor, it is harder to treat because we are looking at multiple sites in the body and working with new tumours that may be resistant to treatment. Discovering that a cancer has spread is always devastating news for patients and their families and so we are delighted to have supported this incredible research which may one day allow us to prevent metastasis and turn cancer into a much more survivable disease."
More information: Richard Gilbertson, The NALCN channel regulates metastasis and nonmalignant cell dissemination, Nature Genetics (2022). DOI: 10.1038/s41588-022-01182-0
Journal information: Nature Genetics
Provided by Cancer Research UK