Researchers discover dozens of genetic defects important for immune defense and relevant for patients with rare diseases
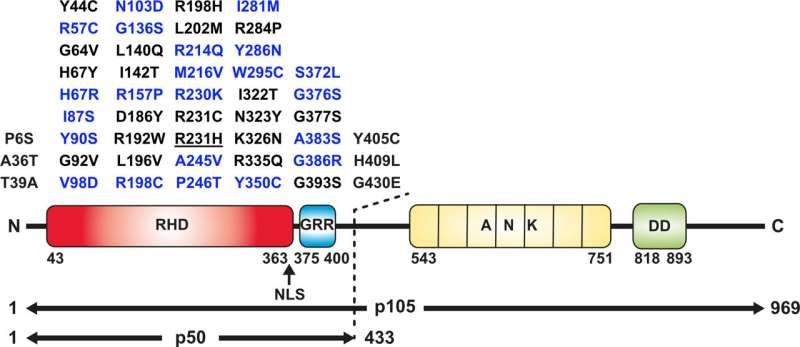
Researchers from the Institute of Biotechnology, University of Helsinki, pioneers in identifying the first patient mutations on the NFkB1-gene, cooperated with international clinicians to identify and characterize a plethora of unreported NFKB1 variants on patients with immune system related illnesses.
In many cases, the identification of a genetic defect in a patient is of great importance for the treatment and prognosis of patients with rare diseases. NFKB1, a transcription factor, causes changes in gene expression and is activated by stress and immune related signaling pathways. Mutations in the NFkB1 have previously been linked to common variable immune deficiency (CVID).
Two new studies published in Frontiers in Immunology may bring further relief for patients with hereditary gene defects in their immune systems.
"These studies have significantly expanded the associations of NFKB1 variants to immune system dysfunction—the connections which we first reportedin 2017," says research director Markku Varjosalo from the Institute of Biotechnology, University of Helsinki
Researchers identified two new NFKB1 variants in two families suffering from common variable immune deficiency. Both identified NFKB1 variants caused reduced expression of the NFKB1 protein and lead to an altered gene expression and increased inflammation response in patient cells. Interaction analysis again showed loss of interactions for one of the variants but not the other.
Another group of researchers studied a cohort of 47 NFKB1 mutations previously reported in patients, out of which 25 did not appear to behave differently from wild type NFKB1. The other 22 mutations were found to have adverse effects on NFKB1 which ranged from increased NFKB1 protein degradation, reduced DNA binding of NFKB1 to overall reduced NFKB1 function through altered protein structure: This may hint towards their likely pathogenicity. Analysis of NFKB1 variant protein interactions showed varied effects from loss of interaction with NFKB family proteins to some variants interactions appearing similar to wild type NFKB1.
"These projects are excellent examples of fruitful international multidisciplinary research collaborations between the University of Helsinki and the leading clinical research hospitals and centers in Europe. Our findings significantly deepen the understanding of the molecular mechanisms underlying NFKB1, and other autoinflammatory and autoimmune diseases associated with altered NFkB1 expression or function," says Varjosalo.
"Also, our results yet again suggest that targeted inhibition of certain key NFkB signaling pathway components is an attractive therapeutic approach for treating these diseases which could be collectively paraphrased as diseases of NF-kB signaling."
More information: Manfred Fliegauf et al, Detrimental NFKB1 missense variants affecting the Rel-homology domain of p105/p50, Frontiers in Immunology (2022). DOI: 10.3389/fimmu.2022.965326
Frederik Staels et al, Common variable immunodeficiency in two kindreds with heterogeneous phenotypes caused by novel heterozygous NFKB1 mutations, Frontiers in Immunology (2022). DOI: 10.3389/fimmu.2022.973543





















