Genetic variants linked to congenital urinary tract obstruction in males
Genetic variation affecting developmental genes not previously linked to urethral development may contribute to a congenital condition that is the most common cause of kidney failure in young males, a study published today in eLife suggests.
The discovery may help scientists better understand what causes a rare condition called posterior urethral valves (PUV), which affects 1 in 4,000 males and leads to blockages in the urethra and a build up of urine in the bladder which can then damage the kidneys. About one-third of individuals with this condition develop kidney failure before age 30. Affected individuals often undergo surgery to remove the blockages, but most continue to have urinary tract problems even after surgery. Therefore, new insights about the condition's cause are required to better understand how the urinary tract develops in health and disease and potentially inform new treatment approaches in the future.
"PUV does not follow a Mendelian pattern of inheritance, where each parent contributes one of two possible alleles for a trait, and scientists have not identified a single gene cause," explains lead author Dr. Melanie Chan, who conducted the study as a Clinical Research Fellow at the UCL Department of Renal Medicine, London, U.K. "This suggests that the genetic basis of this condition is more complex."
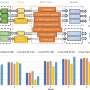
To identify the genetic causes, Chan and colleagues analyzed the genomes of 132 unrelated males with PUV and 23,727 individuals without the condition who had been recruited to the U.K.'s 100,000 Genomes Project. They included individuals with diverse genetic ancestry, including people of South Asian, African and European descent. They found two genetic variants associated with the risk of PUV. One was a common genetic variant located on chromosome 12q24.21 and the other was a rare genetic variant on chromosome 6p21.1. They confirmed the link between these two genetic differences and the disease in a separate group of individuals of European descent that included 395 males with PUV and 4,152 individuals without the condition.
The team then mapped the variation on 12q24.21 to a gene called TBX5, which contributes to turning other genes on or off. They also mapped the 6p21.1 variation to a gene called PTK7, which plays an essential role in cell development. When they looked at cells from developing human embryos, they found that the proteins encoded by the genes are active in the developing urinary tract. This discovery suggests that alterations in these proteins may interfere with normal urethra development.
Finally, they showed that structural changes in chromosomes, including flipped sections of DNA or other changes that alter the regulation of gene expression, were also linked to PUV.
"Our study is the first to identify rare and common genetic variation strongly associated with PUV, as well as structural variations in chromosomes that may contribute to the disease," says Dr. Chan. "It provides new insights on what causes this poorly understood disorder."
The authors add that the small number of individuals included in this genetic analysis reduces its statistical power to detect very rare genetic variations linked with PUV. Additionally, they say more studies are needed to verify how exactly these genetic changes cause PUV.
But senior author Professor Daniel Gale, the St Peter's Chair of Nephrology at the UCL Department of Renal Medicine, says the study demonstrates the importance of including people with diverse genetic backgrounds in genome-wide studies of rare conditions. Too often, he noted, genetic studies may consist of only European populations, making them less likely to identify genetic variants that might be important in other groups.
"Increasing diversity in genetic studies is both scientifically and ethically beneficial," Professor Gale says. "It increases the power of studies to find and verify rare genetic variants and allows detection of genetic variants disproportionately affecting individuals with Asian, African, or other non-European ancestries. It also helps to ensure that people across the world benefit more equally from treatment advances driven by genetic discoveries."
More information: Melanie Mai Yee Chan et al, Diverse ancestry whole-genome sequencing association study identifies TBX5 and PTK7 as susceptibility genes for posterior urethral valves, eLife (2022). DOI: 10.7554/eLife.74777