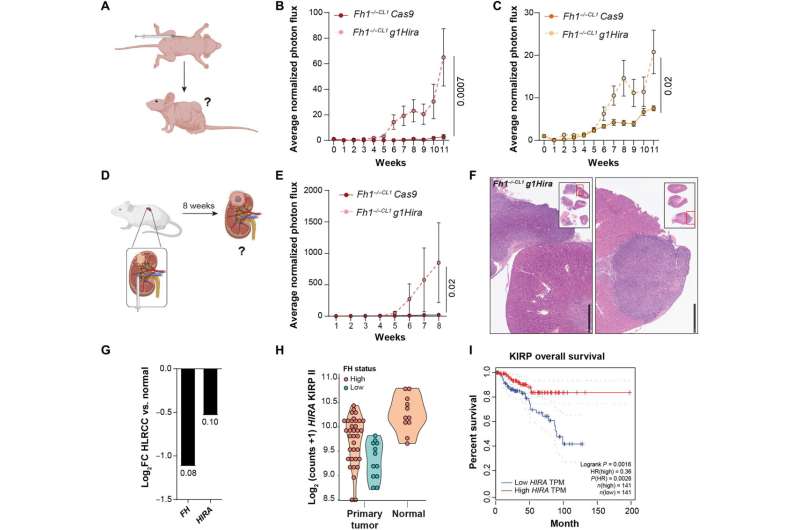
Hira- and Fh1-deficient cells promote tumor initiation, growth, and invasion in vivo.(A) Scheme of xenograft injections in the flank of nude mice. Two million cells were injected in each flank (5 mice, 10 injections in total), and tumor initiation/growth was monitored for 11 weeks by In Vivo Imaging System (IVIS) bioluminescence imaging (BLI). (B and C) Xenograft tumor growth by means of average BLI and flux intensity normalized to day 0 (n = 10 tumors) of Hira- and Fh1-deficient cells (Fh1−/−CL1 g1Hira and Fh1−/−CL19 g1Hira). (D) Scheme of orthotopic experiments carried out. Cells were injected in the kidney capsule (n = 4 kidneys per condition). Tumor initiation, growth, and invasion were analyzed for 8 weeks by IVIS BLI. (E) Representation of average luminescence signal by means of BLI and flux intensity normalized to day 1 (day after surgery) of Fh1-deficient (Fh1−/−CL1 Cas9) and Hira- and Fh1-deficient cells (Fh1−/−CL1 g1Hira). (F) Representative hematoxylin and eosin images of the kidney injected with Hira- and Fh1-deficient cells. Tumors attached to the kidney capsule and invasive lesions within the kidney can be observed. Small square represents the whole sections of the kidney and adjacent tumors. Scale bars, 1 mm. (G) Analysis of FH and HIRA expression data from patients with HLRCC. Numbers on the bars represent P values. (H) Gene expression of HIRA in KIRP II comparing normal and primary tumor samples. Tumor samples with low FH expression are represented in blue. (I) Overall survival data associated to HIRA expression from KIRP using Gene Expression Profiling Interactive Analysis (GEPIA). Low/High HIRA represents top and bottom 50%. Error bars represent SEM. Statistic test performed: two-tailed Mann-Whitney U test. Numbers represent P value for all comparisons. TPM, transcripts per million, HR, hazard ratio; FC, fold change. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abq8297
Deletion of the HIRA protein promotes the proliferation and invasion of tumor cells in cell culture and animal models. That is the result of a research endeavor led by Alexander von Humboldt Professor Dr. Christian Frezza at the University of Cologne.
The hereditary leiomyomatosis and renal cell carcinoma syndrome (HLRCC) is based on a change in the gene that produces the protein fumarate hydratase (FH). Fumarate hydratase is a cell protein that is significantly involved in energy production in cells. However, the mutation in the gene alone does not lead to the disease.
Other factors such as the reduced production of HIRA are necessary for cancer to develop, which the scientists discovered with the help of CRISPR/Cas9 screening in cells. The research, which was started at the MRC Cancer Unit of Cambridge University and completed at the CECAD Cluster of Excellence for Ageing Research at the University of Cologne, has now been published in Science Advances.
"Based on these new mechanistic findings, a model for HLRCC kidney tumors can be developed that opens up new perspectives for targeted therapies," said Dr. Lorea Valcarcel, first author of the study.
In addition to the laboratory experiments, the scientists demonstrated a reduction in FH and HIRA proteins in affected HLRCC patients compared to samples from healthy individuals. Down-regulation of HIRA is also further confirmed by tumor biopsies from two patients compared to the normal surrounding tissue.
Thus, loss of HIRA activates the protein MYC, a known oncogene. Oncogenes are genes and proteins that positively influence tumor formation through its function and activation of other proteins.
More information: Lorea Valcarcel-Jimenez et al, HIRA loss transforms FH -deficient cells, Science Advances (2022). DOI: 10.1126/sciadv.abq8297
Journal information: Science Advances
Provided by University of Cologne