Credit: Pixabay/CC0 Public Domain
Monoclonal antibody therapies continue to neutralize SARS-CoV-2 variants currently in circulation, including omicron BA.4/5, according to laboratory findings from the Francis Crick Institute and the National Institute for Health Research (NIHR) UCLH Biomedical Research Center, published as a research letter in The Lancet.
In the U.K., monoclonal antibodies are part of an important strategy to protect clinically vulnerable people from becoming severely ill with COVID-19. But the WHO recently issued a recommendation against use of all currently-available monoclonal antibodies in the latest issue of its "Living Guideline for Therapeutics and COVID-19."
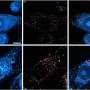
In their study, the researchers sought to test whether monoclonal antibodies might still be effective, using robust high-throughput viral neutralization assays, developed at the Crick. They tested the ability of monoclonal antibodies (sotrovimab, casivirimab, imdevimab, tixagevimab and cilgavimab) to block entry of the virus into cells and "neutralize" different subvariants of SARS-CoV-2, including omicron BA.2, BA.2.12.1, and BA.4/5.
The researchers then looked to a recent analysis of NHS medical records using the OpenSAFELY platform, which showed that one of these monoclonal antibodies, sotrovimab, was superior to treatment with the antiviral drug molnupiravir in preventing hospitalization and death, in clinically vulnerable people during the BA.2 wave.
The team also observed that sotrovimab neutralized omicron BA.4/5 and BA.2.12.1 to a greater extent than omicron BA.2 in the lab, which suggests it would remain effective in clinical use against these two variants.
Credit: The Francis Crick Institute
The team at the Crick and UCLH are proposing that due to the rapid evolution of SARS-CoV-2, recommendations for the use of targeted mononclonal antibody treatments for COVID-19 should be based on regular evaluation of both high-quality, standardized live-virus neutralization data, and efficacy data from real-world clinical use.
Emma Wall, UCLH Infectious Diseases consultant and Senior Clinical Research Fellow for the Legacy study, said, "Living with COVID-19 means different things for different people, and it's essential that we continue to protect the most vulnerable patients with treatment strategies that are based on robust scientific and clinical data.
"It takes a long time for new treatments to be approved for clinical use, but comparatively little time to withdraw them. With the pandemic continuing to evolve rapidly, we need to take a more strategic approach to recommendations for these clinically valuable drugs."
David LV Bauer, Group Leader of the Crick's RNA Virus Replication Laboratory and member of the G2P-UK National Virology Consortium, said, "Our data strongly suggest that we should be more aggressive in getting monoclonal antibodies into the clinic to treat COVID-19.
"Different drugs have been shown to be more or less effective against new variants, when compared to previous strains. But the pace at which the virus is evolving means we shouldn't dismiss potentially effective treatments. Continued monitoring and analysis of emerging variants is our best defense against the virus and essential to protect vulnerable people, like those undergoing dialysis or with blood cancer."
More information: Mary Y Wu et al, WHO's Therapeutics and COVID-19 Living Guideline on mAbs needs to be reassessed, The Lancet (2022). DOI: 10.1016/S0140-6736(22)01938-9
Journal information: The Lancet
Provided by The Francis Crick Institute