'Prime and spike' nasal vaccine strategy helps combat COVID
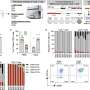
![IN boosting with stabilized SARS-CoV-2 spike induces mucosal humoral memory. (A) Experimental schema: mice were intramuscularly (IM) immunized with 1 μg of mRNA-lipid nanoparticles (LNP) encoding full-length SARS-CoV-2 (SCV2) spike protein (Pfizer/BioNTech BNT162b2), followed by intranasal (IN) immunization with 1 μg of prefusion-stabilized (Hexapro), trimeric, recombinant SCV2 spike protein 14 days following mRNA-LNP immunization. Fourteen days post IN boost, serum, bronchoalveolar lavage fluids (BALF), and nasal washes were collected to assess binding and neutralizing antibody responses. Lung tissues were collected for extravascular B cell analysis. (B to G) Measurement of SCV2 spike S1 subunit-specific nasal wash IgA (B), nasal wash IgG (C), BALF IgA (D), BALF IgG (E), serum IgA (F), and serum IgG (G) in naïve mice, mice immunized with mRNA-LNP IM (IM Prime), mice immunized with the spike protein IN (IN Spike), or mice IM primed and IN boosted with spike (P&S). (H to K) Measurement of neutralization titer against SCV2 spike-pseudotyped vesicular stomatitis virus (VSV) in BALF (H) and serum (I). (J to N) Using CD45 intravenous (IV) labeling, various extravascular (IV labeling antibody negative) B cell subsets were measured, including RBD tetramer-binding B cells, IgA+ resident memory B (BRM) cells, IgG+ BRM cells, IgA+ antibody-secreting cells (ASC), and IgG+ ASC in lung tissues from IM Prime or P&S mice. Mean ± s.e.m. Statistical significance was calculated by [(B) to (G)] one-way ANOVA or [(H) to (N)] Student’s t test; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Individual data points are represented and are pooled from two or three independent experiments. Credit: Science (2022). DOI: 10.1126/science.abo2523 'Prime and spike' nasal vaccine strategy helps combat COVID](https://scx1.b-cdn.net/csz/news/800a/2022/prime-and-spike-nasal.jpg)
A Yale-designed nasal vaccine can help bolster immune responses to COVID-19 in previously vaccinated animals and reduce viral transmission, Yale researchers report Oct. 27 in the journal Science.
The new vaccine approach developed by Yale researchers—which is known as "prime and spike"—is designed to jumpstart immune response in the respiratory system, which is the first part of the body to be infected by the virus.
Intramuscular vaccine shots, which are what most people have received to protect against COVID-19 infection, provide a broad-based immune response throughout the body and help avert serious illness. However, that protection has tended to wane after about four months, leaving people susceptible to breakthrough infections and emerging variants.
The new "prime" and "spike" approach may help prevent breakthrough infections of vaccinated individuals by bolstering immune response within the mucosal lining of the respiratory tract, which are the first cells attacked by COVID-19. ("Prime" refers to the process of injecting the vaccine directly into the muscle, as is typically done. "Spike" refers to a follow-up vaccination with spike proteins, known to derive from the coronavirus, directly into the nostril, where the virus is known to enter the body.)
"The nasal vaccine promotes the development of immunity within the respiratory system, which can respond more quickly to infection," said Dr. Benjamin Goldman-Israelow, assistant professor of medicine (infectious diseases) at the Yale School of Medicine and co-corresponding author of the paper. "By creating mucosal immunity, the vaccine helps stop the virus at its entry point, rather than waiting until later to fight back."
The Yale team, led by Goldman-Israelow and Tianyang Mao, a graduate student in the lab of fellow co-corresponding author Akiko Iwasaki, Sterling Professor of Immunobiology, delivered the nasal vaccine to both vaccinated and unvaccinated mice. They found increased immune system response in the respiratory tracks of vaccinated mice only. Mice that had not been previously vaccinated, and vaccinated mice that did not receive nasal vaccine, died from infection. Meanwhile, mice that received the intranasal spike booster were completely protected from death and disease.
In hamsters that received the nasal vaccine, the Yale team found decreased duration and total amount of viral shedding, which is when an infected individual releases copies of the virus including through sneezing or coughing. Hamsters vaccinated with the prime and spike method were also found to have reduced viral loads when housed in the same cage with infected, unvaccinated hamsters, suggesting that the strategy reduces trains of transmission.
Researchers also found increased breadth of immune response among animals that received the nasal vaccine, indicating that the approach would be effective against a broad spectrum of coronaviruses with pandemic potential.
Goldman-Israelow noted that the Yale approach does not use live viruses, viral vectors, or adjuvants, which may make the vaccine safer.
More information: Tianyang Mao et al, Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses, Science (2022). DOI: 10.1126/science.abo2523