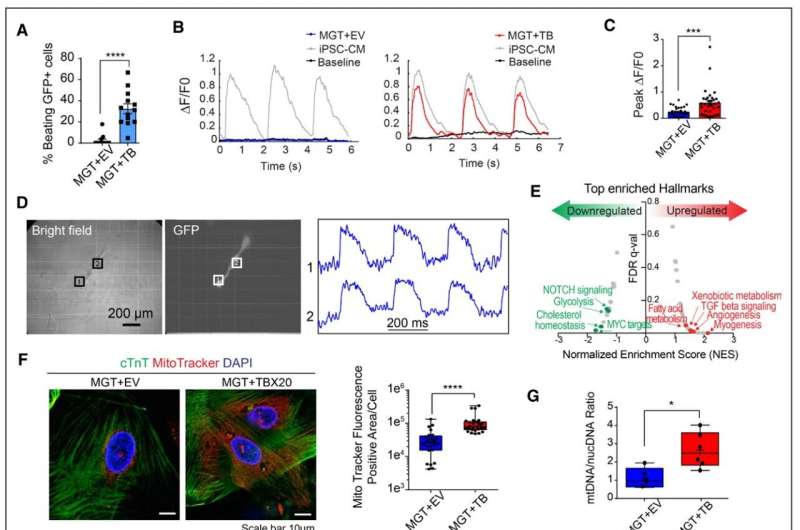
TBX20 overexpression leads to functional improvement of hiCMs. A, Quantification of observed beating human induced cardiomyocytes (hiCMs) derived from H9-derived fibroblasts (H9Fs) after 1 month of co-culture with human induced pluripotent stem cell–derived cardiomyocytes (iPSC-CMs; n=10 for each group, repeated 2 times independently). B, Tracing and measurements from microscopy imaging show calcium transients in MGT + empty vector (EV) or MGT+TBX20 co-cultured cells as indicated in A. C, Quantification of measured peak ΔF/F0 in co-cultured hiCMs (n=33, repeated 2 times independently). D, Bright field and GFP (green fluorescent protein) fluorescence images showing MGT+TBX20-induced individual hiCMs after co-culture. Right panel shows the selected optical traces of membrane potential (Vm) during spontaneous beating with a cycle length of 270 ms. E, Gene set enrichment analysis of RNA sequencing data from H9F-derived hiCMs showing the enriched hallmarks in TBX20 upregulated and downregulated genes. F, Representative images and quantification of mitochondria content labeled using MitoTracker in cardiac troponin T (cTnT)+ hiCMs derived from H9F (n=20 per group). Scale bars, 10 μm. G, Reverse transcription quantitative polymerase chain reaction results showing the relative ratio of mitochondria DNA to the total genomic DNA in H9F-derived hiCMs (n=6 per group). H, Representative oxygen consumption rate profile analyzed from mito-stress Seahorse assay in hiCMs derived from H9Fs (n=10 per group, repeated 3 times independently). I, Quantification of basal respiration, maximal respiration, proton leak, spare respiratory capacity, adenosine triphosphate (ATP) production, and spare respiratory capacity as a percentage in hiCMs treated with MGT+TBX20 or MGT+EV from H9Fs (n=10 per group). J, Representative extracellular acidification rate profile analyzed from glucose-stress Seahorse assay in TBX20-treated or EV-treated hiCMs derived from H9Fs (n=10 per group). K, Quantification of glycolysis, glycolytic capacity, and glycolytic reserve in H9F-derived hiCMs transduced with TBX20 or EV (n=10 per group). All data are expressed as mean ± SEM (A, C, F, G, I, and K, Student t test). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. ECAR indicates extracellular acidification rate; FDR, false discovery rate; and OCR, oxygen consumption rate. Credit: Circulation (2022). DOI: 10.1161/CIRCULATIONAHA.122.059713
Mammalian hearts have almost no ability to grow new heart muscle cells, called cardiomyocytes, after birth. Thus, dead tissue after an adult heart attack is not repaired with new cardiomyocytes. It is instead replaced with scar tissue that weakens the pumping power of the heart and often leads to heart failure.
One promising strategy to remuscularize the injured heart is the direct cardiac reprogramming of heart fibroblast cells into cardiomyocytes. In a paper published in Circulation, University of Alabama at Birmingham researchers Yang Zhou, Ph.D., and Rui Lu, Ph.D., have identified TBX20 as the key missing transcription factor in existing cocktails for direct cardiac reprogramming of human fibroblasts.
Adding TBX20 to the reprogramming cocktail MGT 133, they report, promoted cardiac reprograming and activated genes associated with cardiac contractility, maturation and a ventricular location of the heart muscle cell. Mechanistically, they found that TBX20 synergistically colocalizes with the MGT reprogramming factors at cardiac gene enhancers, causing robust activation of target genes.
"Our study highlights the undocumented role of TBX20 as a vital regulator of direct human cardiac reprogramming," Zhou said. "Enhancing the efficiency and quality of direct cardiac reprogramming from human fibroblasts is a critical step in the clinical translation of this technology."
An important area for future investigation, Zhou says, will be testing TBX20 in vivo for direct reprogramming.
Current cocktails for direct reprogramming of human fibroblasts suffer from low efficiency and insufficient production of functional cardiomyocytes. The UAB researchers identified TBX20 as the most underexpressed factor when they compared cardiomyocytes induced from fibroblasts with a current cocktail versus functional cardiomyocytes.
The addition of TBX20 promoted cardiac reprogramming, as seen in activation of cardiac genes related to sarcomere structure, ion channels and heart contractions. A sarcomere is the smallest functional unit of striated muscle.
Human heart fibroblasts of the ventricle will be the primary target for evaluation of in vivo reprogramming. Zhou and colleagues showed that TBX20 was essential in reprogramming these fibroblasts to induced-cardiomyocytes, suggesting a therapeutic potential for TBX20.
In detail, TBX20 primarily activated genes at the late stage of reprogramming, enhancing calcium flux, contractility and mitochondrial function in the induced-cardiomyocytes. Mitochondria are the energy source for heart muscle contractions. TBX20 appeared to help the mitochondria in the induced-cardiomyocytes switch to an adult cardiomyocyte-like respiration.
Mechanistically, the UAB researchers found that TBX20 was bound to and activated cardiac gene enhancers. An enhancer is a short regulatory element on DNA that can bind activator proteins to initiate transcription or increase the transcription of a particular gene target. Transcription is the production of messenger RNA copied from the DNA gene to encode a protein. Enhancers often function at a distance from its gene target.
This enhancer binding by TBX20, Zhou and colleagues found, required all three of the MGT cocktail factors to help activate cardiac genes. TBX20 alone was not sufficient to induce cardiac cell fate conversion; omission of any of the three MGT factors substantially reduced reprogramming efficiency.
"Together, our findings demonstrate that TBX20 requires intact MGT factors to promote direct reprogramming and activate cardiac genes, and they support the notion that direct cardiac reprogramming is a synergistically regulated process orchestrated by multiple factors," Zhou said.
Single-cell RNA sequencing profiling identified two major clusters of cells after reprogramming with TBX20. One was reprogrammed induced-cardiomyocytes, but the other group was only partially reprogrammed. The cluster of reprogrammed cardiomyocytes had gene expression profiles resembling ventricular cardiomyocytes.
More information: TBX20 improves contractility and mitochondrial function during direct human cardiac reprogramming, Circulation (2022). DOI: 10.1161/CIRCULATIONAHA.122.059713
Journal information: Circulation
Provided by University of Alabama at Birmingham