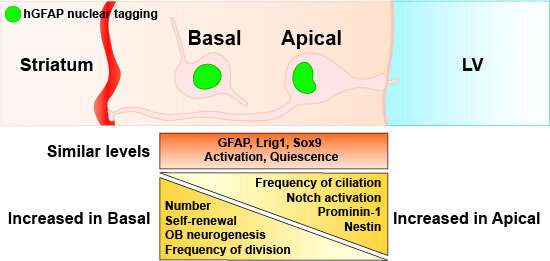
Basal neural stem cells are the most abundant stem cell type in the adult mouse V-SVZ from birth onwards and the main contributors to olfactory bulb neurogenesis. Credit: EMBO reports (2022). DOI: 10.15252/embr.202154078
In the brain of adult mammals, neural stem cells ensure that new nerve cells (neurons), are constantly formed. This process, known as adult neurogenesis, helps mice maintain their sense of smell. A research team led by Dr. Francesca Ciccolini at the Interdisciplinary Center for Neurosciences (IZN) of Heidelberg University recently discovered a second stem cell population in the mouse brain. This new type of stem cell is primarily involved in the production of new neurons in the olfactory bulb of adult mice.
Until now, scientific investigations on neurogenesis have concentrated on the so-called apical stem cells. "They were long considered to be the only stem cell population in the adult mouse brain as well as the main driver of nerve cell formation," explains Dr. Ciccolini. These neural stem cells are located in the subventricular zone near the lateral cerebral ventricle.
They were once believed to form the precursor cells that then differentiate in the olfactory bulb of mice into interneurons, nerve cells that modulate stimuli transmission between interconnecting neurons. The Heidelberg researchers were able to disprove the single stem cell type theory and the assumption that apical stem cells are responsible for neurogenesis.
Originally, the researchers in the Neurobiology Department were investigating how this allegedly lone stem cell population in the mouse brain behaves in various situations. They used genetically modified animals whose neural stem cells were dyed green using a dye active in the cell nucleus.
The neurobiologists were surprised to discover that most of the green cells did not display the known characteristics of apical stem cells. "At first, we thought that they could be astrocytes, helper cells that ensure that the neurons are able to do their work. But after we conducted a number of function analyses, it rapidly became clear that these had to be a separate stem cell population," says Dr. Ciccolini.
Further studies showed that the newly discovered stem cell type differs from the known population in its morphology as well as its function. This type of cell has no contact with the lateral cerebral ventricle and is therefore called basal. The researchers determined that the basal—and not the apical—stem cells are responsible for the formation of neurons in the olfactory bulb.
To prove this, they separately labeled both cell populations and then observed whether labeled neurons turned up in the olfactory bulb. "This only happened when the basal population was labeled," explains Francesca Ciccolini. When only apical stem cells were labeled, no new labeled neurons could be detected in the olfactory bulb.
The Heidelberg scientists also found out that both stem cell types and precursor cells in the mouse brain communicate with one another via so-called notch interactions. A receptor of the same name plays a vital role, controlling the speed at which the cells multiply and monitoring the cell differentiation process.
"The notch activity decides, as it were, whether a stem cell remains a stem cell or develops into a nerve cell," explains Katja Baur, a doctoral researcher in Francesca Ciccolini's working group. "We suspect that the apical stem cells intervene in the activation of the notch signal pathway and can inhibit proliferation and neurogenesis," she adds. Amongst other things, that prevents the depletion of the stem cell reservoir.
"Our discovery that another stem cell type exists in the mouse brain of adult animals throws new light on the processes of neuron formation," says Dr. Ciccolini. The human brain has similar stem cells that are involved in the formation of brain tumors. The Heidelberg researchers hope that their work will also shed new light on the development and possible treatment of such tumors.
The research was published in EMBO reports.
More information: Katja Baur et al, A novel stem cell type at the basal side of the subventricular zone maintains adult neurogenesis, EMBO reports (2022). DOI: 10.15252/embr.202154078
Journal information: EMBO Reports
Provided by Heidelberg University