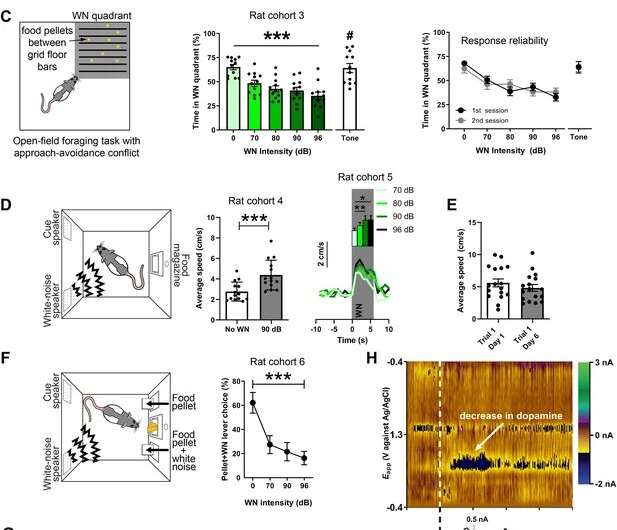
White noise (WN) is an aversive stimulus that lowers dopamine concentration in the nucleus accumbens core (NAC). (A) Left: example trajectory of a rat in the real-time place aversion test (30 min), in which entry into one quadrant (shaded), which was randomly determined prior to the session, led to 90 dB WN exposure. Right: aversiveness of WN in the place aversion test quantified as significantly decreased time spent in the WN quadrant (n = 10, 9.71 ± 1.65, t(9) = 9.585, p<0.0001). (B) Left: a second cohort of rats was placed in the open field to assess their preferred quadrant in a first session. In a second session, the entry into the preferred quadrant led to 90 dB WN exposure. Right: when WN was administered in this quadrant, rats spent significantly less time in it (n = 15, No WN: 48.5 ± 0.2474, 90 dB: 7.983 ± 0.3875, t(14) = 13.43, p<0.0001). (C) Left: a third cohort of rats (n = 12) was exposed to an open-field foraging task in which food pellets were placed between grid-floor bars in one of the quadrants. Entry into the grid-floor quadrant (shaded) led to exposure of 0, 70, 80, 90, or 96 dB of WN, or a 3 kHz tone in separate sessions. Middle: increasing WN intensity dose-dependently decreased the amount of time rats spent foraging for food pellets in the grid-floor quadrant (χ2(5) = 27.6, p<0.0001), whereas the tone did not reduce foraging (70 dB WN vs. 70 dB tone (#); t(10) = 2.389, p=0.0381). Right: the rats’ response to the different WN intensities and the tone was reliable across first and second sessions of exposure, where rats underwent exposure to all WN intensities and the tone in a first session, before exposure to each in a second session. (D) Left: all other experiments took place in operant boxes equipped with a food magazine, a multiple-tone generator (cue speaker), and a WN generator (WN speaker). Middle: semi-random presentations of 6 s WN bouts increased the locomotion speed of rats (cohort 4; n = 14) in an operant box during the WN epoch compared to pre-WN baseline (post-hoc Dunn’s test, t(13) = 7.059, p<0.0001). Right: in another cohort of rats (cohort 5; n = 13), we tested different WN intensities and found a main effect of intensity on locomotion speed (χ2(3) = 13.80, p=0.0032) and significant differences between 70 and 90 dB (p=0.005) and 70 and 96 dB (p=0.0143) (n=13). (E) Rats (n = 17) responded reliably with increased locomotion speed to WN across days. (F) Rats (cohort 6; n = 9) discern between different WN magnitudes in an operant choice task, where they had to choose between pressing a lever that resulted in a food-pellet delivery and a lever that resulted in a food-pellet delivery plus simultaneous 5 s of 0, 70, 90, or 96 dB of WN (pellet + WN; Friedman test, χ2(3) = 11.57, p=0.0003). (G) Left: example cresyl violet-stained brain slice depicting an electrolytic lesion in the NAC (outlined) at the tip of the fast-scan cyclic voltammetry (FSCV) electrode (vertical black line). Right: schematic overview of FSCV recording locations (blue dots) in the NAC (gray) of all animals. (H) Single-trial pseudocolor plot (top panel), dopamine trace (bottom panel), and cyclic voltammograms (inset in bottom panel) for representative, dopamine-specific current fluctuations recorded in NAC, 5 s before WN (dashed line), during 6 s of WN (gray bar), and 14 s after WN. Except for panel (H), data are mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. Credit: eLife (2022). DOI: 10.7554/eLife.82711
A new study at the Netherlands Institute for Neuroscience has examined how the dopamine system processes aversive unpleasant events.
It is well known that the dopamine system plays a crucial role in motivation, learning and movement. One of the main functions of dopamine is to predict the occurrence of rewarding experiences and the availability of rewards in our environment. In this context, the dopamine system informs our brains about so-called "reward prediction errors"—the difference between received and predicted rewards.
Dopamine neurons become more active when a reward occurs unexpectedly or if it is bigger than expected, and they show depressed activity when we receive less reward than predicted. These error signals help us to learn from our mistakes and teach us how to achieve rewarding experiences.
Rewarding versus aversive stimuli
While a large number of studies has focused on the relationship between dopamine release and rewarding stimuli, few have looked at the effect of unpleasant and aversive stimuli on dopamine. Although the results of these few experiments have been inconsistent, it has become clear that aversive stimuli have an impact on the dopamine system.
But there is an active debate among neuroscientists on what precise role dopamine neurons play in processing aversive stimuli: Does their activity change in response to aversive events? Do they predict aversive events? Do they encode an aversive prediction error?
New findings on the role of dopamine in aversive events
Now published in eLife, a new study at the Netherlands Institute for Neuroscience has examined how the dopamine system processes aversive events. The team around Ph.D. student Jessica Goedhoop and group leader Ingo Willuhn exposed rats to white noise in combination with stimuli that predicted the white noise, while they measured the release of dopamine in the brain. White noise is a well-known example of an unpleasant auditory stimulus for rats.
The researchers found that the release of dopamine gradually decreased during the exposure to white noise. Furthermore, after consistent presentation, stimuli that occurred a few seconds before white-noise exposure began to have the same depressing effect on dopamine neurons. However, in contrast to how it processes rewards, dopamine did not encode a prediction error for this aversive stimulus.
Overall, this new study demonstrates that the dopamine system helps the brain to anticipate the occurrence and duration of unpleasant events, but without taking prediction errors into account.
Group leader Ingo Willuhn stated, "This is a very thorough and systematic study that takes a lot of variables into account. The results give us a better understanding of the role of dopamine release in processing aversive events. There is a growing interest into the role of dopamine in aversion. We used a novel aversive stimulus that enabled to conduct a more thorough analysis of dopamine than previously possible."
Addictive drugs hijack and amplify dopamine signals and induce exaggerated, uncontrolled dopamine effects on neuronal plasticity. This study brings us closer to understanding the underlying mechanism behind this pathological phenomenon.
More information: Jessica N Goedhoop et al, Nucleus accumbens dopamine tracks aversive stimulus duration and prediction but not value or prediction error, eLife (2022). DOI: 10.7554/eLife.82711
Journal information: eLife
Provided by Netherlands Institute for Neuroscience