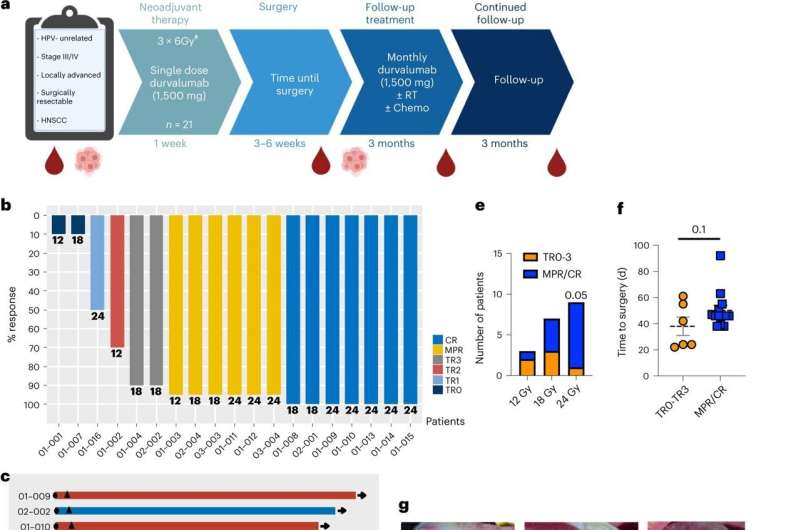
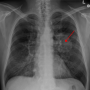
a, Diagram of trial design. b, Summary of pathological outcomes of all evaluable patients (n = 19 patients). c, Summary of clinical outcomes of patients treated at MTD (n = 18). d, Kaplan–Meier survival curves for overall survival, PFS and local PFS survival for patients treated at MTD (n = 18 patients). e, A plot of the relationship between radiation dose and pathologic outcome at time of surgery (P = 0.05), spearman correlation coefficient 0.45, CI (−0.01909, 0.7573). 12 Gy n = 3 patients, 18 Gy n = 7 patients and 24 Gy n = 9 patients. f, Analysis of the relationship between time to surgery and pathological response (mean ± s.e.m.). Pathologic tumor response (pTR) pTR0–3 n = 6 patients and MPR/CR n = 13 patients. g, Representative images of a patient’s tumor response to treatment. h, Representative standardized uptake value (SUV) images showing a decrease in signal intensity post-treatment. A two-sided Fisher’s exact test was used in e. Statistical significance was determined by an unpaired two-sided Student’s t-test for f. Significance was concluded if P was less than 0.05. For the last 9 patients the GTV was dosimetrically calculated at 24 Gy. Credit: Nature Cancer (2022). DOI: 10.1038/s43018-022-00450-6
A new study from researchers at the University of Colorado Anschutz Medical Campus has identified a less invasive way to treat a subset of head and neck cancers, which could potentially change the standard of care for patients.
HPV-unrelated head and neck squamous cell carcinomas (HNSCC) typically do not trigger immune responses and have not responded well to immunotherapies. The current standard of care for these tumors begins with surgery that can require tongue or jaw removal and other facial and oral complications, followed by six weeks of radiation with or without chemotherapy
In a new study, just published in Nature Cancer, the Anschutz researchers detail a different approach to treat these tumors, using radiation combined with immunotherapy to invigorate the patient's immune response and reduce immune exhaustion.
This approach, combined with just one cycle of the drug Durvalumab within a specific pre-operation timeframe primes the immune system to kill most or all of the cancer before surgery. The level of success observed in this preoperative marriage of treatments is remarkable.
Of the 21 HNSCC patients participating in the Phase I/Ib dose-escalation study, major pathological responses or complete responses was 75%, and 89% for those in the expansion cohort who received the optimal radiation dose. And for those on the expansion cohort with MPR/CR, none of them received adjuvant radiotherapy or chemotherapy despite large tumors at presentation and none have recurred to date. Time to surgery was also a critical element in allowing response to be observed and for immune system priming to happen.
"The response in our patients to this dual-treatment protocol surpassed our expectations," says Sana Karam, MD, Ph.D., corresponding author, and member of the CU Cancer Center. "By combining treatment modalities, we were able to spare our patients' lymph nodes and prime their immune systems, ultimately resulting in a less morbid treatment regimen."
In conducting the translational analysis, Anschutz MD/Ph.D. student Laurel Darragh observed a major increase in T-cell infiltration in the tumor and several biomarkers in the blood. The blood correlates could be detected preoperatively such that it was clear pre-surgery who would respond to the treatment. All patients went on to receive 4 additional cycles of postoperative Durvalumab, but those who failed the treatment did so despite the adjuvant treatment.
"Our biggest takeaway from these results is hope for our patients," says Karam. "The response rates here are truly unparalleled, so we are hopeful that this work will lead to a significantly improved standard of care in how we treat these types of cancers."
Researchers hope to move forward with these results to validate potential biomarkers in a larger, ongoing Phase II trial.
More information: Laurel B. Darragh et al, A phase I/Ib trial and biological correlate analysis of neoadjuvant SBRT with single-dose durvalumab in HPV-unrelated locally advanced HNSCC, Nature Cancer (2022). DOI: 10.1038/s43018-022-00450-6
Journal information: Nature Cancer
Provided by CU Anschutz Medical Campus