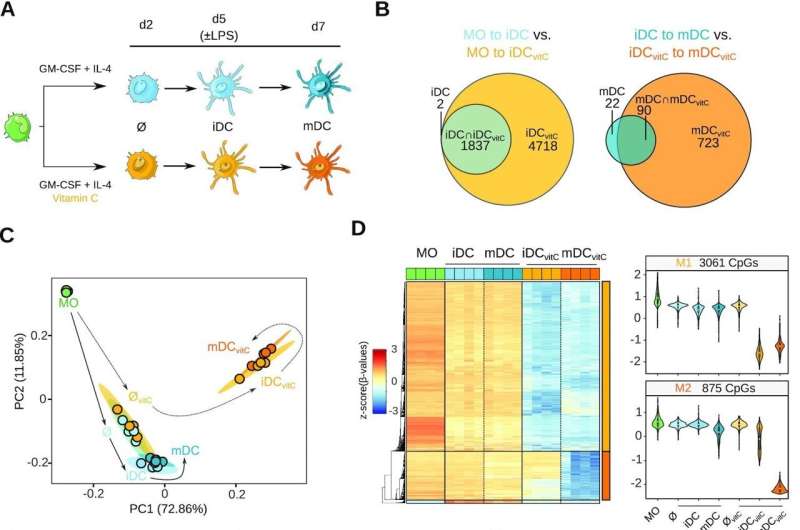
Vitamin C-mediated dendritic cell DNA methylome remodeling. (A) Scheme depicting the experimental setup. Monocytes (MO) were differentiated to dendritic cells (DCs) using GM-CSF and IL-4, in the presence or absence of vitamin C (vitC). Samples were collected on day 2, in the middle of the differentiation, and on day 7, including immature DCs (iDCs) and mature DCs (mDCs), exposed the last 2 days to lipopolysaccharide (LPS). (B) Area-proportional Venn diagrams comparing the demethylated CpG sets of MO-to-iDC versus MO-to-iDCvitC, and iDC-to-mDC versus iDCvitC-to-mDCvitC transitions. (C) Principal Component Analysis (PCA) of differentially methylated positions (DMPs) comparing all groups pairwise. Principal component 1 and principal component 2 are represented on the x- and y-axis, respectively. Solid and dashed lines represent the trajectories across MO-iDC-mDC and MO-iDCvitC-mDCvitC differentiation/maturation respectively, to make the plot easier to follow. (D) DNA methylation heatmap of DMPs comparing iDC to iDCvitC, and mDC to mDCvitC (Δβ ≥ 0.3, FDR < 0.05). Scaled β-values are shown (lower DNA methylation levels in blue and higher methylation levels in red). On the right side, violin plots of clusters M1 and M2 depict scaled β-values. (E) Enrichment of M1 and M2 DMPs in ChromHMM 15-states categories of MOs (Roadmap Epigenomics Project). Fisher's exact tests of M1 and M2 DMPs were calculated using all the CpGs annotated in the EPIC array as background. Significantly enriched categories (FDR < 0.05 and odds ratio > 2) are depicted with a black stroke, including TxFlnk (Flanking Active TSS), TxWk (Weak Transcription), EnhG (Genic Enhancers), and Enh (Enhancers). (F) GO (Gene Ontology) over-represented categories in M1 and M2 DMPs. Fold Change in comparison with background (EPIC array CpGs) and –log10(FDR) is represented. (G) Bubble scatterplot of transcription factor binding motif enrichment for M1 and M2 DMPs. The x-axis shows the percentage of windows containing the motif and the y-axis shows the fold enrichment of the motif over the EPIC background. Bubbles are colored according to the transcription factor family. FDR is indicated by bubble size. Credit: Nucleic Acids Research (2022). DOI: 10.1093/nar/gkac941
Researchers from the Epigenetics and Immune Disease Lab at the Josep Carreras Leukaemia Research Institute have recently shown that vitamin C improves the immunogenic properties of dendritic cells in vitro.
The team's results, recently published in Nucleic Acids Research, show that treating the cells with vitamin C leads to a more consistent activation of genes involved in the immune response, mainly through DNA demethylation, a kind of epigenetic reprogramming. This discovery may be useful for generating more potent dendritic cell-based therapies in the future.
Since the onset of anticancer cell therapies, which use living cells to find and eliminate tumors, many types of immune cells have been used. The best-known cell therapies use lymphocytes, as in highly successful CAR-T therapies.
Recently, dendritic cells have attracted scientists' attention, due to their ability to uptake and present antigens (small parts of a pathogen or a cancer cell) to T-lymphocytes and induce an antigen-specific potent immune activation. In this regard, loading dendritic cells with specific antigens to create immune memory constitutes so-called DC vaccines.
To study dendritic cells in the lab, researchers differentiate them from monocytes (also an immune cell) using a particular type of molecular signaling. This differentiation is accomplished through a complex set of gene activation processes in the nucleus, mostly due to the activity of the chromatin remodeling machinery spearheaded by the TET family of demethylases, proteins that act upon the DNA epigenetic marks.
Vitamin C was known to interact with several TET proteins to enhance its activity, but the specific mechanism was still poorly understood in human cells. In the Nucleic Acids Research study, a team lead by Dr. Esteban Ballestar hypothesized that treating monocytes in vitro during the dendritic cell differentiation process would help the resulting cells be more mature and active.
The results obtained by Octavio Morante-Palacios, first author of the publication, José Luis Sardina (also from the Josep Carreras Leukaemia Research Institute) and Eva Martínez-Cáceres, Head of Immunology of the Germans Trias i Pujol Research Institute, show that vitamin C treatment triggers an extensive demethylation at NF- kB/p65 binding sites compared with non-treated cells, promoting the activity of genes involved in antigen presentation and immune response activation. Vitamin C also increases the communication of the resulting dendritic cells with other components of the immune system and stimulates the proliferation of antigen-specific T cells.
Furthermore, the researchers proved that vitamin C-stimulated dendritic cells loaded with antigens specific to the SARS-CoV-2 virus more efficiently activated T cells in vitro than did non-treated cells, showing the superiority of DC vaccines treated with vitamin C.
Overall, these new findings support the hypothesis that treating monocyte-derived dendritic cells with vitamin C may help generate DC vaccines with higher performance. After consolidating these results in preclinical models, and hopefully in clinical trials, a new generation of cell therapies based on dendritic cells could be used in the clinic to fight cancer more efficiently.
More information: Octavio Morante-Palacios et al, Vitamin C enhances NF-κB-driven epigenomic reprogramming and boosts the immunogenic properties of dendritic cells, Nucleic Acids Research (2022). DOI: 10.1093/nar/gkac941
Journal information: Nucleic Acids Research
Provided by Josep Carreras Leukaemia Research Institute