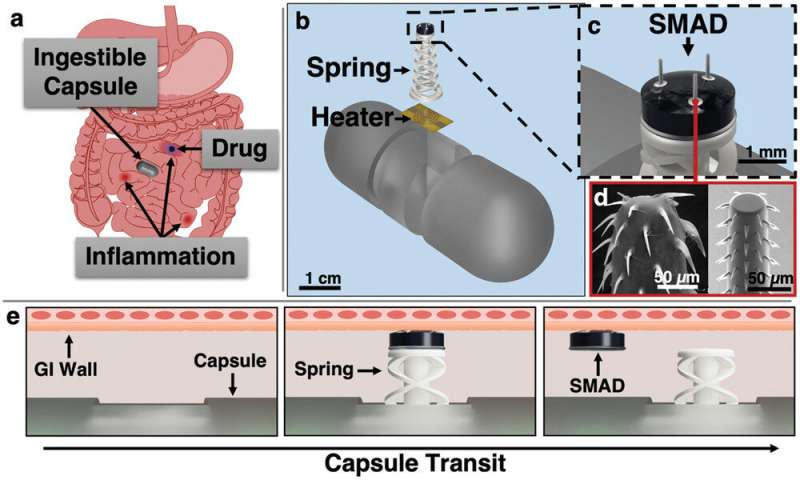
Actuation and SMAD delivery principle. a) Capsule transits through a GI tract with inflammatory lesions and delivers the SMAD to an inflammatory site for prolonged release of a topical therapeutic agent (blue). b) Actuator deployment utilizes a resistive heating element to fire a spring actuator and impart the drug-loaded SMAD into the GI tissue. c) CAD rendering of the SMAD on the actuator. d) Spiny microneedles demonstrated previously are designed to mimic a spiny-headed worm proboscis for enhanced anchoring in tissue: Reproduced under terms of the CC-BY license. e) Upon sensed or external command for SMAD delivery, current passes through the heating element, melting the polycaprolactone binder, and firing the spring. The SMAD is then imparted into the tissue and removes as the capsule translates through the intestinal tract. Credit: Advanced Materials Technologies (2022). DOI: 10.1002/admt.202201365
A 3D printed drug-delivery system using a sensor, heating element and wave spring sounds like a machine wheeled into the doctor's office, not something popped in your mouth.
Yet a University of Maryland engineering team has developed a futuristic new capsule that can be ingested, guided and activated to detect, monitor and treat chronic problems in the gastrointestinal tract.
This new research, published this week in the journal Advanced Materials Technologies, demonstrates a tiny spring actuator that can anchor the capsule, allowing it to deliver a drug deposit to planned locations in the GI tract. With the ability to stay in place for a sustained period of time, the capsule can deliver multiple doses of medication as needed.
"Our innovation is an early example of using hybrid fabrication approaches that merge 3D printing with traditional microfabrication to create new and impactful devices," said first author Joshua Levy, a materials science and engineering doctoral student. "We expect our work will help form the foundation of new forms of treatment, and that these devices eventually will lead to better therapies."
He is part of Professor Reza Ghodssi's (ECE/ISR) MEMS Sensors and Actuators Laboratory, which has been working on capsule development for five years. Others in the A. James Clark School of Engineering who contributed to the research included bioengineering doctoral student Michael Straker, electrical and computer engineering doctoral student Justin Stine, UMD research associate Luke Beardslee, electrical and computer engineering alum Vivian Borbash '22 and Ghodssi. All of the graduate students are associated with the Robert E. Fischell Institute for Biomedical Devices.
Some 3.1 million people in the United States suffer from chronic GI autoimmune disorders like inflammatory bowel disease, Crohn's disease and ulcerative colitis. Medical science has made substantial advances in the last few decades, largely through "systemic" therapies like pills, injections and infusions. Unfortunately, as these therapies diffuse throughout the body, their effectiveness also diminishes. Medicine can't be targeted to the inflammatory lesions that characterize these gut diseases, and the treatments produce substantial side effects.
"The GI tract is a passage through the body that influences who we are through its direct connections to the outside world," Ghodssi said. "This unique organ is susceptible to a number of health grand challenges, from cancer to IBD to neurodegenerative diseases, and mental health problems and metabolic diseases as well."
Capsules can perform GI imaging, gas sensing, lesion biopsy and drug delivery, and they can be commanded remotely through Wi-Fi and a phone app. Still, one problem has persisted: how to keep the capsule in place to deliver medicine amid the constant churning of the digestive system.
This new research introduces the thermomechanical 3D-printable spring actuator, a mechanism that works with existing ingestible capsule-based sensing and communication technologies and enables treatment based on detected GI biomarkers and external commands, which can be delivered via Bluetooth.
The actuator is combined with the first application of the Ghodssi's biomimetic barbed microneedle technology, known as SMAD, for Spiny Microneedle Anchoring drug Deposit. When it's time to deploy the spring and propel its payload of therapeutic drugs, the capsule's tiny resistive heating element melts a material called polycaprolactone that holds it in place. The SMAD is then released from the spring to provide prolonged dissolving therapeutic drug delivery to specific lesions.
"We hope that our emerging noninvasive capsule technology will be able to put another tool in the medical kit, one with fewer side effects and better targeted efficacy," Ghodssi said. "Our work addresses only one of the promising research areas for this technology. We believe developing ingestible capsules is a frontier of research that requires an interdisciplinary team of doctors, engineers, biologists and data analysts to solve."
More information: Joshua A. Levy et al, Thermomechanical Soft Actuator for Targeted Delivery of Anchoring Drug Deposits to the GI Tract, Advanced Materials Technologies (2022). DOI: 10.1002/admt.202201365
Journal information: Advanced Materials Technologies
Provided by University of Maryland