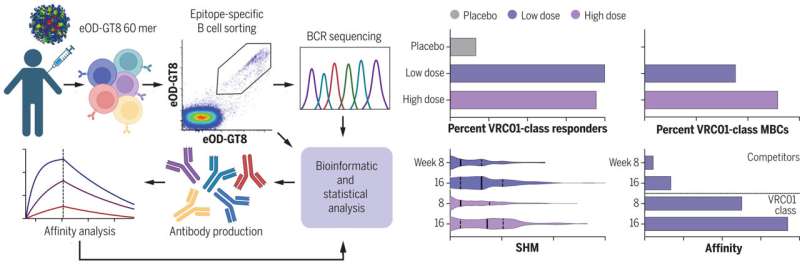
Test of germline-targeting vaccine priming in healthy humans. Immune cells were isolated from recipients of eOD-GT8 60mer vaccine or placebo, and antibody sequences from vaccine-binding B cells were analyzed to measure the VRC01-class bnAb-precursor response rate among participants and the frequency of VRC01-class bnAb-precursor B cells among memory B cells (MBCs) in each participant. Somatic hypermutation (SHM) and binding affinity were measured. Credit: Christopher Cottrell, Created with Biorender.com, Science (2022). DOI: 10.1126/science.add6502
A large team of researchers affiliated with a host of institutions across the U.S., working with two colleagues from Sweden, reports promising results in a phase I clinical trial aimed at testing the efficacy and safety of an HIV vaccine.
In their paper published in the journal Science, the group describes using a germline-targeting priming immunogen approach in developing the vaccine and how well it performed during its initial clinical trial. Penny Moore, with the National Institute for Communicable Diseases, in South Africa, has published a Perspectives piece in the same journal issue outlining germline targeting in vaccines and the work done by the researchers on this new effort.
Medical researchers have been working for decades on developing an HIV vaccine, but unfortunately they have yet to succeed. The problem, as the researchers with this new effort note, is that the virus mutates so quickly. By the time a vaccine is developed, the virus has changed in ways that allow it to overcome the new vaccine. And that has led researchers to try to develop vaccines containing B-cells that prompt the body to generate broadly neutralizing antibodies that bind with parts of the virus that do not change much when it mutates.
In a new strategy, the researchers developed a protein called gp120 that binds to a unique part of the surface of the HIV virus—a part that helps the virus make its way into human cells. Their work showed that this part of the virus does not change during mutations. They then added other materials that prompt the immune system into producing B-cells that have the gp120 protein.
After initial testing in the lab, the researchers put together a phase I clinical trial to test whether the vaccine incited the immune system to create the antibodies as hoped. They recruited 48 volunteers, none of whom were HIV positive, to take part in the trial—36 of them received the new vaccine, while 12 served as a control.
Each of the volunteers gave weekly blood samples for 16 weeks. At the end of the trial, 35 of the volunteers who received the vaccine generated the desired antibodies—and none of them reported any serious side-effects. More testing is required to determine if the antibodies produced due to the vaccine actually prevent HIV infections.
More information: David J. Leggat et al, Vaccination induces HIV broadly neutralizing antibody precursors in humans, Science (2022). DOI: 10.1126/science.add6502
Penny L. Moore, Triggering rare HIV antibodies by vaccination, Science (2022). DOI: 10.1126/science.adf3722
Journal information: Science
© 2022 Science X Network