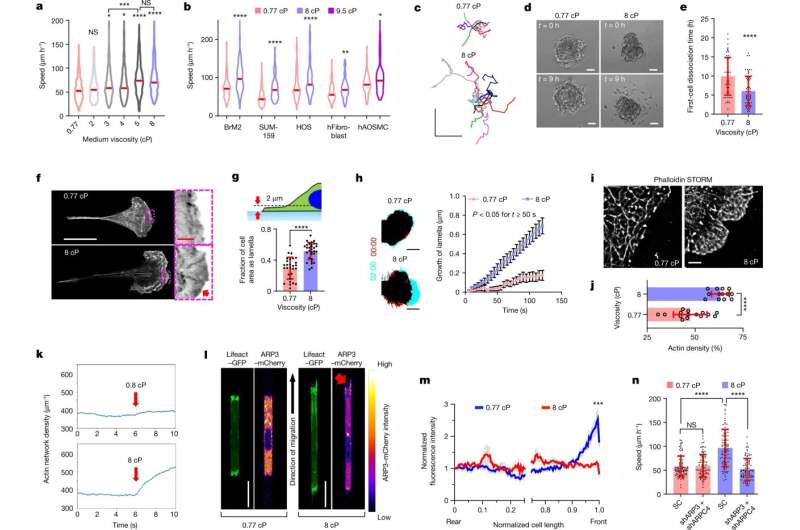
Viscosity enhances cell migration and promotes an ARP2/3-mediated dense actin network at the leading edge. a,b, Speeds of MDA-MB-231 cells (a) and other indicated cell types (b) inside confining channels at prescribed viscosities. The red lines represent the median of ≥69 cells from ≥3 experiments. c, Cell trajectories on 2D collagen-coated surfaces after 10 h. d, Cells disseminating from 3D spheroids. e, The time required for the first cell dissociation from each spheroid (n ≥ 53) from 3 experiments. f, Airyscan images of phalloidin stained cells on collagen-coated substrates. The red arrow indicates high F-actin staining along the cell edge. g, The fraction of cell-projected area with a Lifeact–GFP-rich lamella for n ≥ 28 cells from 3 experiments. h, The leading edge of Lifeact–GFP-expressing cells on collagen-coated surfaces at t = 0 min (red) and t = 2 min (cyan) (left). Right, leading-edge lamella growth in n ≥ 19 cells from 3 experiments. Data are the moving average ± s.e.m. P < 0.05 for all points t ≥ 50 s. Time is shown as min:s. i,j, STORM reconstruction (i) and density quantification (j) of F-actin for cells (n ≥ 13) on substrates from 2 experiments. k, The average actin density over time from 20 stochastic simulations. Viscous forces were applied at t = 6 s (red arrow) and maintained until the end of the simulation. l, Confocal images of cells expressing Lifeact–GFP and ARP3–mCherry in confinement. The red arrow indicates high ARP3 intensity at leading-edge protrusions at 8 cP. m, The relative ARP3–mCherry intensity along normalized cell length in confined cells. Data are the moving average ± s.e.m. for n = 21 cells from 4 experiments. ***P < 0.001 for all comparisons at normalized cell length > 0.96. The x axis is discontinued between 0.25 and 0.75 to highlight differences at the cell edges. n, Confined migration speeds of SC versus ARP3/ARPC4 double-knockdown cells (n = 90) from 3 experiments. For e, g, j and n, data are mean ± s.d. Unless otherwise indicated, statistical comparison was performed with respect to 0.77 cP. Statistical analysis was performed using Kruskal–Wallis tests followed by Dunn’s test (a and n), Mann–Whitney U-tests (BrM2 only) or unpaired t-tests after log-transformation (other cells) (b), unpaired t-tests (e, g and j) and two-way analysis of variance (ANOVA) followed by Šidák’s test (h and m). Scale bars, 250 μm (c), 50 μm (d), 25 µm (f, white), 3 µm (f, red), 10 µm (h), 2 µm (i), 20 µm (l). The cell model was MDA-MB-231 unless otherwise indicated. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Credit: Nature (2022). DOI: 10.1038/s41586-022-05394-6
An international team of researchers has uncovered a new mechanism that enables cancer cells to move throughout the body, providing a potential new target to stop metastasis, which is responsible for 90 percent of cancer deaths.
In findings published in Nature, the team identifies that cancer cells move faster when they are surrounded by thicker fluids, a change that occurs when lymph drainage is compromised by a primary tumor.
"This is really the first time that the viscosity of the extracellular fluid has been looked at in detail," says John D. Lewis, professor and Bird Dogs Chair in Translational Oncology at the University of Alberta's Faculty of Medicine & Dentistry. "Now that we know that fluid viscosity signals cancer cells to move in a specific way, we can potentially use drugs to basically short-circuit that signaling pathway and encourage cancer cells to slow down, or even maybe to stop."
The Lewis lab was invited to join the project led by researchers at Johns Hopkins University, because of its expertise in imaging human cancer cells in real-time motion using the placenta-like chorioallantoic membrane from fertilized chicken eggs.
"I would say we're the world leaders in this type of imaging," Lewis says. "Our contribution to the work was to very precisely show that cancer cells change their gene expression when they encounter increased viscosity in the surrounding fluid and become more aggressive. And even when you bring the viscosity back down, these cells stay more aggressive."
"We then went on to show that when this signaling pathway is perturbed in cancer cells it changes their ability to escape the bloodstream and metastasize," Lewis says.
This is the third paper the international research team has published. Lewis credits Konstantin Stoletov, senior research associate, for the bulk of his team's work. He cautions that once a new therapeutic target is identified, it could take 10 to 15 years to develop and test a drug.
"But this is helping us build our understanding around how cancer cells move and it increases our chance of being successful with this whole approach," he explains.
More information: Kaustav Bera et al, Extracellular fluid viscosity enhances cell migration and cancer dissemination, Nature (2022). DOI: 10.1038/s41586-022-05394-6
Journal information: Nature
Provided by University of Alberta