Credit: Unsplash/CC0 Public Domain
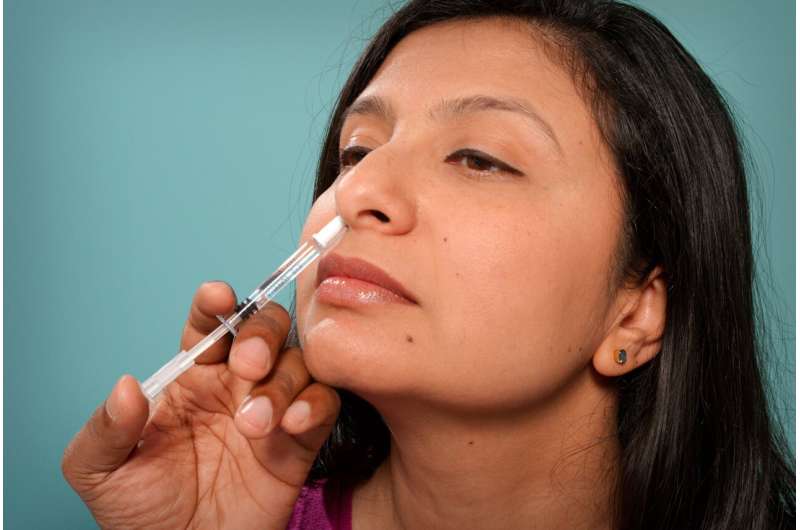
Messenger RNA vaccine technology, once an arcane area of research, became household terminology because of the COVID-19 pandemic, and now scientists are working on an mRNA flu vaccine strategy that, at least in this study, involves a first dose administered as a shot but a booster administered as an mRNA nasal spray.
The vaccine is known as a self-amplifying mRNA vaccine that targets influenza's viral nucleoprotein. That structure in influenza A viruses is a highly conserved multi-functional protein and is a key target in vaccine and antiviral research because it is less likely to mutate compared with viral surface proteins.
The strategy devised by researchers, primarily at the University of Minnesota who are working with collaborators elsewhere in the United States, was to generate lung-resident memory T cells that are stably maintained in respiratory tissues.
So far, the research is underway in animal models but the hope is to craft a vaccine—and method of vaccine administration—that helps conquer one of humankind's most persistent foes: the flu.
Each year in the United States, an estimated 36,000 deaths and millions of hospitalizations are due to influenza-related illness. Globally, the World Health Organization estimates 250,000 to 500,000 flu deaths annually. And in the case of influenza pandemics, the viral infection causes even more catastrophic damage as was the case in 1918 when an estimated 500 million people, about one-third of the world's population, became infected with the virus and 50 million died.
The goal in the Minnesota-based research was an mRNA flu vaccine strategy that prompts resident memory T cells to fan out in the lungs and to be prepared in the event of infection.
"Respiratory tract resident memory T cells, typically generated by local vaccination or infection, can accelerate control of pulmonary infections that evade neutralizing antibody," writes Dr. Marco Künzli of the Center for Immunology in the Department of Microbiology and Immunology at the University of Minnesota.
"It is unknown," Künzli added, "whether mRNA vaccination establishes respiratory resident memory T cells."
As the lead author of the study, which was published in Science Immunology, Künzli and a large team of scientists, wanted to know if they could do just that—help establish respiratory resident memory T cells in the lungs of animal models via mRNA vaccine technology.
The strategy they devised for the research—which worked—involved an initial prime and boost with intramuscular vaccination followed by a secondary intranasal booster. This strategy efficiently promoted memory CD4 and CD8 T cells in lung tissue. Beyond the current research, the findings help highlight how mRNA vaccine technology can be adapted to protect the lungs from any respiratory infection, for example, such as RSV—respiratory syncytial virus—or beyond RSV, any other pathogen from a vast swath respiratory infectious agents.
While intramuscular prime and boost immunizations were sufficient to induce respiratory resident memory T cells in the animal models, an additional intranasal boost further expanded both circulating and lung resident memory T cells, the researchers found.
"We generated a self-amplifying mRNA vaccine encoding the influenza A virus nucleoprotein," Künzli continued, noting the nucleoprotein "is encapsulated in modified dendron–based nanoparticles."
"We report how routes of immunization in mice, including contralateral versus ipsilateral intramuscular boosts, or intravenous and intranasal routes, influenced influenza-specific cell-mediated and humoral immunity," Künzli asserted.
Scientists left no stone unturned in their research. After the multi-pronged administration strategy, they checked the respiratory resident memory T cells' functions in the mice through parabiosis, a surgical protocol commonly used in immunological studies involving the respiratory resident memory T cell subpopulation.
Initial analysis demonstrated that respiratory resident memory T cells insinuated themselves in lung tissue becoming long-term residents after the prime-boost intramuscular administration. The intranasal booster, which further increased the number of respiratory resident memory T cells, helped establish memory CD4 and CD8 T cells, circulating and residing in the lungs.
The new research, which shows potential for new ways to administer an mRNA to achieve an immunological goal, arrives following the success of mRNA vaccines against SARS-CoV-2.
Scientists are also moving ahead with research on mRNA vaccines for a large number of diverse medical disorders, including various forms of cancer, rare diseases, and yes, numerous infectious diseases. The new influenza research underscores that mRNA technology is not only robust but capable of being explored as a highly tailored weapon against the flu.
"The prospects are exciting for combating emerging pathogens, antigenically variable pathogens that might be addressed by megavalent vaccination, and perhaps non-infection conditions such as personalized tumor vaccines," Künzli noted.
More information: Marco Künzli et al, Route of self-amplifying mRNA vaccination modulates the establishment of pulmonary resident memory CD8 and CD4 T cells, Science Immunology (2022). DOI: 10.1126/sciimmunol.add3075
Journal information: Science Immunology
© 2022 Science X Network