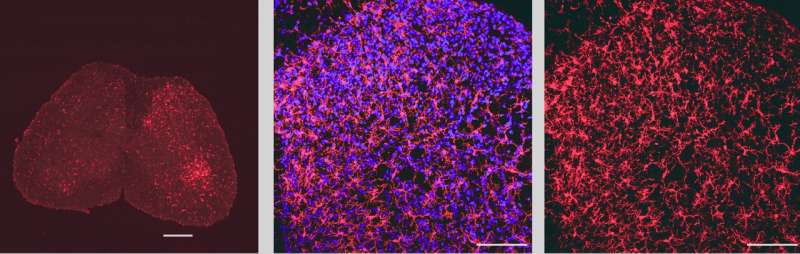
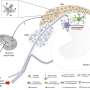
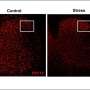
Image showing microglia in the lumbar spinal cord labeled with the microglial marker IBA1 (in red). The blue stain shows nuclei of cells labeled with a nuclear marker. Credit: Prakirya laboratory
Northwestern Medicine investigators have discovered that specific calcium channels help regulate sex differences in the functioning of immune cells for neuroinflammation and overall neuropathic pain, according to findings published in Science Advances.
"This study highlights the importance of microglial Orai1-mediated calcium signaling contributing to neuropathic pain. It provides a foundation for us to continue to investigate what role Orai1 plays in microglial reactivity," said Kaitlyn DeMeulenaere, a student in the Driskill Graduate Program in Life Sciences (DGP) and co-first author of the study.
Murali Prakriya, Ph.D., the Magerstadt Professor of Pharmacology and a professor of Medicine in the Division of Allergy and Immunology, was senior author of the study.
Microglia are the primary immune cells of the brain and resemble macrophages found in the peripheral immune system. Microglia detect and clear harmful pathogens and dying cells and stimulate other immune cells that ignite effective immune responses in the brain. In addition to their surveillance functions, in healthy brains, microglia also influence the formation of synapses, or connections, between neurons, that impact neural circuits and regulate overall cognitive functions.
On the other hand, microglia can also cause persistent neuroinflammation and neuropathic pain, including chronic pain caused by nerve injury, and produce pro-inflammatory cytokines that cause uncontrolled neuroinflammation. Microglia may also drive long-lasting changes in synaptic circuits in the central nervous system to enhance the sensation of pain, according to Prakriya.
"The signals in the spinal cord that normally do not activate pain circuits are altered in such way that after nerve injury, the same signals are relayed through the spinal cord to higher brain somatosensory brain structure. This change is mediated in part by inflammatory mediators, cytokines that spinal microglia produce," said Prakriya, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Prakriya's laboratory discovered a class of calcium channels called Orai1 channels more than a decade ago and since then has continued to investigate their critical role in activating immune cells.
In the current study, Prakriya's team aimed to determine what role Orai1 plays a role in microglial activation and neuropathic pain following nerve injury.
Using microglial Orai1 knockout mouse models, they found when Orai1 function was blocked or deleted, Orai1-mediated calcium signaling was lost, ultimately reducing the production of inflammatory cytokines.
"This an important finding because it implicates Orai1 as a checkpoint, if you will, in the cascade of events that leads to the production of inflammatory cytokines that drive pain hypersensitivity," Prakriya said.
Next, the investigators compared measures of pain hypersensitivity following sciatic nerve injury in mouse models. Surprisingly, they identified distinct differences in pain hypersensitivity in male mice versus in female mice that lacked Orai1.
Specifically, they found that loss of Orai1 mitigated pain hypersensitivity in male mice, but not in female mice. This prompted the investigators to further investigate the causes of the sexual dimorphism of pain perception.
Using spinal cord electrophysiology techniques, they found that in male microglial Orai1 knockout mice, the potentiation, or an increase in strength, of synaptic transmission that occurs following nerve injury was reduced. Male mice also showed reduced induction of inflammatory cytokines in spinal cord tissue in response to nerve injury, however this was not observed in female mice.
"There is no change in neuropathic pain perception in female mice because there's no change in the maladaptive synaptic potentiation that occurs downstream of the inflammatory cytokines produced by microglia. Because the maladaptive synaptic potentiation is likely the critical step that drives neuropathic pain, it explains why there is mitigation in male but not female mice," Prakriya said.
Using a microglial marking technique called IBA1 staining, the investigators found that although microglial activation was increased in both male and female mice equally, inhibited Orai1 function reduced the extent of microglial activation in male but not female mice.
They then gave both male and female mice a small-molecule Orai1 inhibitor compound called CM4620, which is currently being tested in clinical trials, and found that while the drug mitigated neuropathic pain in male mice, it had no effect in the female mice.
The findings demonstrate that Orai1 channels are key mediators of microglia-induced neuroinflammation and the sexually dimorphic role of microglia in neuropathic pain, underscoring the importance of developing sex-specific targeted therapies in the future.
"This study points to striking sex differences in the underlying mechanisms mediating pain hypersensitivity between males and females. We are now working on identifying whether Orai1 signaling can be manipulated in other immune cells to provide pain relief in female mice," Prakriya said.
More information: Shogo Tsujikawa et al, Regulation of neuropathic pain by microglial Orai1 channels, Science Advances (2023). DOI: 10.1126/sciadv.ade7002
Journal information: Science Advances
Provided by Northwestern University