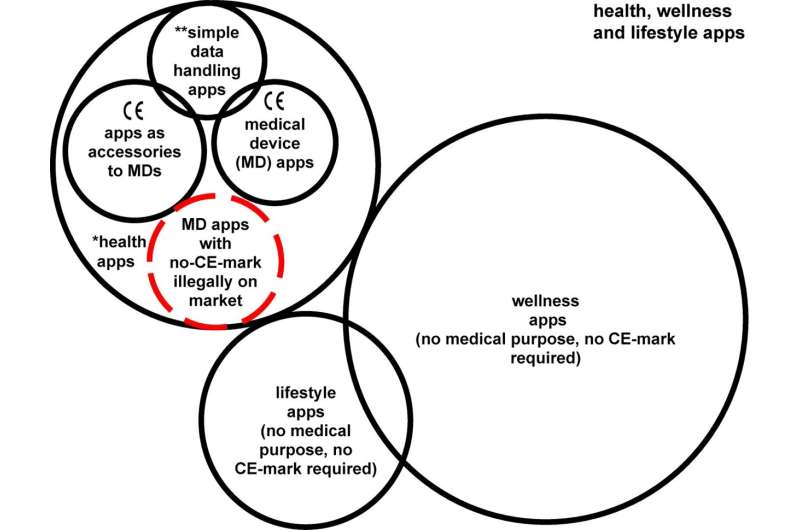
Categories of apps in the health, wellness, and lifestyle area. MD Medical Devices, CE Conformité Européenne. *health apps: this group includes apps that must be CE-marked, and simple data handling apps (**) for the transfer, communication, compression, storage, conversion, formatting, archive, display or simple search of medical information, which must only be CE-marked if they have overlapping MD functionalities. Credit: npj Digital Medicine (2023). DOI: 10.1038/s41746-023-00754-6
Stephen Gilbert and colleagues published an article in npj Digital Medicine on the conflicts of interest faced by app store operators Google and Apple, which are both legal distributors and importers of health apps into the European market and developers of those same apps.
Google and Apple dominate as a "duopoly" with their app stores. They distribute and import many thousands of health and wellness apps into the EU, some of which are approved as medical devices. New ones are added daily. Under EU rules, those who distribute health apps are also responsible for ensuring that they meet certain regulatory requirements.
"There are great products and responsible developers, but there are also irresponsible actors providing tools that are neither safe nor comply with basic regulatory requirements. Google and Apple provide every medical app to every user, be they a patient or a health care provider. With great power comes great responsibility. Thus far, the two tech giants are fulfilling their compliance oversight duties in a patchwork fashion, in the case of Apple, or minimally, in the case of Google. They need to do better, but their ability to fulfill their legal duties is compromised by stark conflicts of interest," said Prof. Stephen Gilbert, Professor of Medical Device Regulatory Science at the Else Kröner Fresenius Center for Digital Health at Technische Universität Dresden.
The EU, in the 2017 Medical Devices Regulation, introduced requirements not only for app developers, but also for app store operators, who have a legal role of distributors. Since then, these have had to ensure that apps comply with medical device regulations in the EU and inform the authorities about serious incidents related to their use.
"Under the 2022 EU Digital Markets Act they will have to walk a tightrope between enforcement and oversight and making sure they do not act unfairly to the apps for which they are the duopoly platform providers. This calls into question the sustainability of a model in which a crucial aspect of health care infrastructure, namely app stores, is provided in such a duopolistic and conflicted manner. The EU has powers to legally enforce changes to this model. Finally, with the proposed European health data space regulation, wellness apps will be voluntarily registered and labeled in a fashion more like medical devices than consumer software."
The authors explore the implications of these new regulations and propose alternative models that could resolve the apparent conflicts. "To enable the health and wellness app sector to further develop safely, these 'wild west' aspects of the market must be resolved. All stakeholders would benefit from improve app store models to sustainably evolve safer, better, and fairer provision of digital health applications in the EU. As EU legislation comes into force it could serve as a template for other regions globally," concludes Gilbert.
More information: Olamide Sadare et al, Can Apple and Google continue as health app gatekeepers as well as distributors and developers?, npj Digital Medicine (2023). DOI: 10.1038/s41746-023-00754-6
Journal information: npj Digital Medicine
Provided by Dresden University of Technology