January 18, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study shows how iron dysregulation might contribute to neurodegenerative diseases
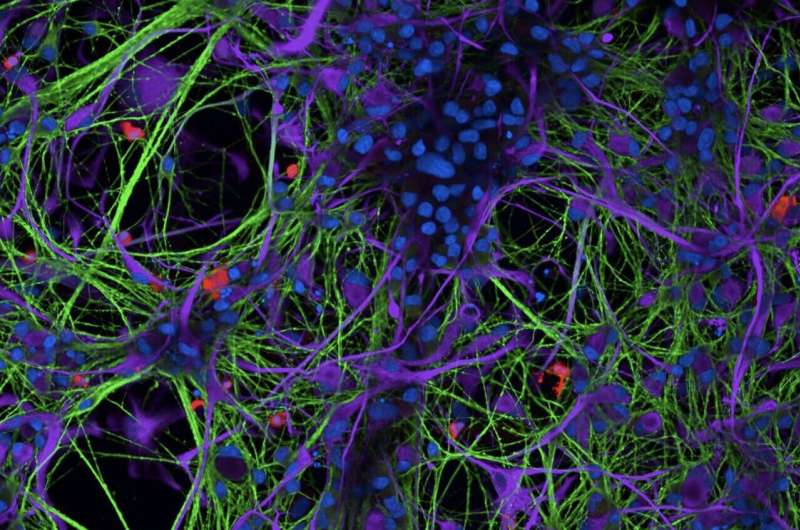
Past neuroscience research consistently found a link between deviations from the "normal" iron metabolism, also known as iron dysregulation, and different neurodegenerative diseases, including Parkinson's disease (PD) and Multiple Sclerosis (MS). Specifically, brain regions associated with these diseases have been found to be often populated by microglia (i.e., resident immune cells) packed with Iron.
While the association between iron dysregulation and neurodegenerative diseases is well documented, the ways in which iron accumulation affects the physiology of microglia and neurodegeneration are yet to be fully grasped. Researchers at global health care company Sanofi have recently carried out a study aimed at filling this gap in the literature, by better understanding how microglia respond to iron.
"For years it has been known that iron accumulates in affected brain regions in PD, MS and other neurodegenerative diseases," Timothy Hammond, one of the researchers who carried out the study, told MedicalXpress. "This is something we can see in patients using MRI imaging, where it has been shown that iron levels increase over the course of the disease. We also had our own data from progressive MS patients showing iron dysregulation in brain microglia, the resident immune cells of the brain."
The key objective of the recent work by Hammond and his colleagues was to better understand how iron accumulation in microglia affects these cells' functioning and health. Their work builds on their previous studies, and on the 2012 discovery of an iron-dependent form of cell death, known as ferroptosis.
Ferroptosis is a form of cell death that is mediated by iron-dependent lipid peroxidation, a process that damages lipids by oxidizing them. In their paper, the researchers' hypothesized that iron-laden microglia are susceptible to ferroptosis and that this might play a role in neurodegenerative diseases.
"We had to leverage several approaches in this study, including single cell transcriptomics and CRISPR, but the tool that really allowed us to tease apart these mechanisms was a complex tri-culture of human iPSC-derived cells containing microglia, astrocytes, and neurons—three of the major cell types in the brain," Hammond explained. "This tool was previously developed by a scientist on my team, Sean Ryan, who is also the lead author of our paper."
To conduct their experiments, the researchers grew microglia in a tri-culture system. Using a series of genetic and experimental techniques, they then showed that these microglia are highly responsive to iron and also susceptible to ferroptosis.
In addition, the team showed that an overload of iron causes a shift in the microglial transcriptional state, which overlaps with a transcriptomic signature observed in microglia in brain tissue from deceased patients with PD. When they removed microglia from their tri-culture system, Hammond and his colleagues observed that iron-induced neurotoxicity in the system significantly slowed down. This suggests that microglia responses to iron overload play a crucial role in neurodegeneration.
This study is one of the first to show how iron-laden microglia might contribute to different PD and other neurodegenerative diseases. In the future, it could pave the way for new important discoveries, potentially informing the development of new therapeutic interventions for these diseases.
"We believe microglia are actually trying protect neurons by taking up toxic iron and storing it safely, but over time the microglia become overwhelmed and die, releasing the stored iron and causing the neurons to die too," Hammond said. "We have to be careful how we target microglia therapeutically, because they perform lots of beneficial activities, but if we can find a targeted approach this could be an important node for diseases like Parkinson's with excessive iron accumulation."
More information: Sean K. Ryan et al, Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration, Nature Neuroscience (2022). DOI: 10.1038/s41593-022-01221-3
Jonathan D. Proto et al, Disrupted microglial iron homeostasis in progressive multiple sclerosis, bioRxiv (2021). DOI: 10.1101/2021.05.09.443127
© 2023 Science X Network