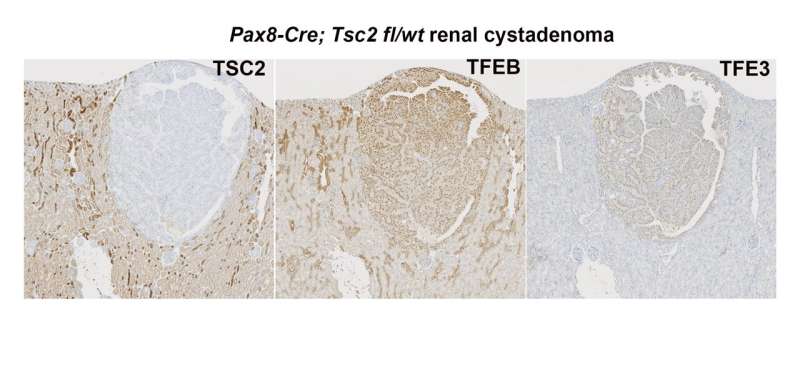
Murine renal tumors with Tsc2 loss have increased TFEB/TFE3 nuclear localization. Credit: Clarence Rachel Villanueva and Adrianna A. Mendes, Nature Communications (2022). DOI: 10.1038/s41467-022-34617-7
In a new study from the Johns Hopkins Kimmel Cancer Center, researchers described a novel mechanism of tumor formation in kidney cancers driven by overexpression of the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway with loss of the tuberous sclerosis complex (TSC) tumor suppressor gene. Their findings point to potential therapeutic targets for some of the most aggressive renal cell cancers.
Unopposed signaling—overactivity—through mTOR may lead to abnormal activation of a family of molecules that regulate cell growth and spread, also known as oncogenic transcription factors, specifically the microphthalmia transcription factor family (MITF).
The findings were reported Nov. 10 in Nature Communications.
Genitourinary cancer expert and study leader Tamara Lotan, M.D., professor of pathology and oncology with a joint appointment in urology, has studied mTOR signaling in embryonic development and cancer for more than a decade. Although mTOR has been the focus of many cancer studies for its role in regulating cell division and growth, its mechanisms in promoting cancer are not fully understood, Lotan says.
"For years, the dogma has been that mTOR signaling directly suppresses the activity of the microphthalmia-related transcription factors TFEB and TFE3 by keeping them out of the nucleus and unable to activate transcription," Lotan says. "However, we found that TFEB and TFE3 are actually, paradoxically, activated downstream of abnormal, continuous mTOR signaling."
The protein mTOR is important in cancer cell phosphorylation, or the activation of proteins involved in cell cycle, including cell death, DNA repair and more; and Lotan says continuously activated mTOR signaling is common in renal and other types of tumors that are prone to losing the tumor suppressor genes TSC1 or TSC2.
"A better understanding of these mechanisms is what makes this work particularly interesting," says Kaushal Asrani, M.B.B.S., Ph.D., a research associate in the Lotan laboratory. "We have known for some time that there are subsets of kidney and soft tissue tumors that can be caused by overactive mTOR signaling or other genetic alterations that directly activate TFEB and TFE3, but how these molecular events were all tied together was a mystery. This work suggests that the unifying mechanism in all cases is activation of TFEB and TFE3."
These transcription factors are typically inactivated in response to cellular nutrients such as amino acids. In laboratory experiments, Asrani found that this amino acid-dependent regulation of TFEB and TFE3 is actually suppressed in kidney tumor cells with TSC loss, leading to their overactivity. The combined loss of TFEB and TFE3 was enough to reduce growth of tumors with continuous mTOR signaling.
The new findings are informing renal cell cancer research while also improving the understanding of other cancers, including pancreatic cancer and melanoma skin cancer, Lotan says.
She is hopeful that the findings may point to new targeted therapies for kidney cancers with overactive mTOR, which will be a focus of ongoing research.
More information: Kaushal Asrani et al, An mTORC1-mediated negative feedback loop constrains amino acid-induced FLCN-Rag activation in renal cells with TSC2 loss, Nature Communications (2022). DOI: 10.1038/s41467-022-34617-7
Journal information: Nature Communications
Provided by Johns Hopkins University School of Medicine