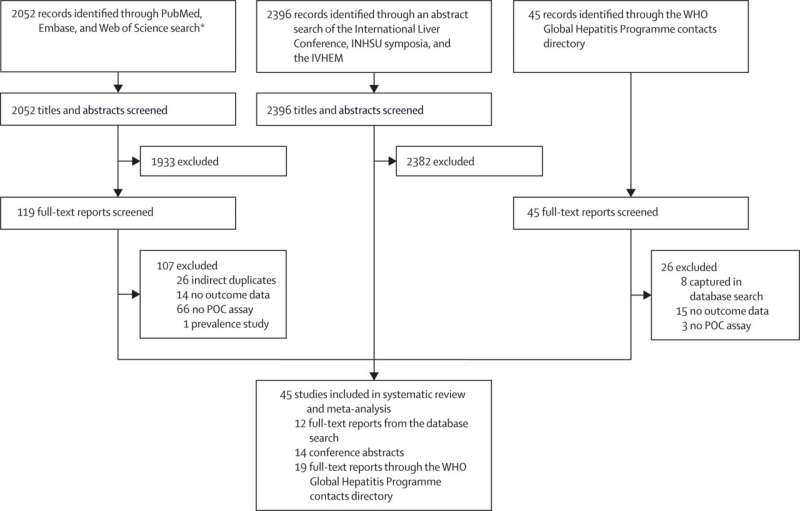
Study selection. AASLD=American Association for the Study of Liver Diseases. APASL=Asia-Pacific Association for the Study of the Liver. INHSU=International Network on Hepatitis in Substance Users. IVHEM=International Viral Hepatitis Elimination Meeting. POC=point-of-care. *AASLD and APASL conference abstracts were captured in Embase database search. Credit: The Lancet Gastroenterology & Hepatology (2023). DOI: 10.1016/S2468-1253(22)00346-6
A new study has shown the benefit of using a quick clinic-based diagnostic test for hepatitis C virus (HCV) infection over a standard laboratory-based test. The findings from the study has led to World Health Organization (WHO) guidelines recommending the adoption of the point-of-care (POC) HCV test.
Chronic hepatitis C infection is a major global public health problem and a cause of liver disease, with the highest burden in low-and middle-income countries (LMICs). The research led by the WHO in collaboration with the University of Bristol is published in The Lancet Gastroenterology and Hepatology.
The study pooled results from 45 studies (around 50% were from LMICs) and compared POC HCV viral load tests with centralized, laboratory-based standard approaches. It showed that using POC HCV viral load tests led to faster time from initial HCV antibody screening to starting on treatment (19 days compared to 67 days).
Overall uptake of treatment was higher with POC tests at the clinic site (77%) or delivered in mobile units (81%) compared with standard laboratory-based tests (53%). The best results were seen when the POC tests were placed at a site where both testing and treatment were offered—a "one stop shop" enabling treatment to be started on the same day as diagnosis.
Philippa Easterbrook of Global Hepatitis Program at WHO headquarters and senior author, commented, "WHO is encouraging affected countries to consider including use of point-of-care assays in their national policies on hepatitis C. The COVID-19 pandemic prompted a major expansion of these point-of-care diagnostic assays in many low- and middle-income countries, which provides an opportunity to share their use for hepatitis C viral load testing and therefore reduce costs.
"Many countries including Australia, Cambodia, Malaysia and Myanmar have adopted use of these point-of-care assays in their national programs. WHO is now undertaking a similar evaluation of point-of-care viral load assays for hepatitis B in its planned 2023 updated guidance."
Dr. Adam Trickey, research fellow in the Bristol Medical School: Population Health Sciences (PHS) at the University of Bristol and an author of the paper, added, "The POC test can be placed in harm-reduction clinics, primary care clinics, prisons, or in mobile units or even community clinics, and offers the possibility of a one-stop shop same-day diagnosis and treatment of HCV infection. These POC tests can be used to get people diagnosed and onto hepatitis C treatment more quickly, including hard to reach or marginalized populations at high risk of loss to follow-up, such as persons who inject drugs, or homeless persons."
The findings from the study has led to recent WHO recommendations for adoption of POC HCV viral load testing as an alternative approach to laboratory-based platforms for diagnosing HCV infection and speeding up starting treatment for the infection.
This recommendation complements other recent WHO recommendations in the same updated guidance for HCV diagnosis and treatment that promotes radical simplification of service delivery and task-sharing of testing and treatment to nurses and non-specialist doctors.
In 2016, WHO launched the Global Health Sector Strategy for Viral Hepatitis which was renewed in 2022, with a goal of ending the epidemics of viral hepatitis B and C by 2030. Good progress has been made with more than 10 million persons with chronic hepatitis C infection cured using a 12-week short course direct acting antiviral treatment.
However, as of 2019 there were still 58 million people with chronic hepatitis C, resulting in 399,000 deaths. Only 20% of those infected worldwide had been diagnosed and 13% treated. To close this gap and achieve WHO targets for eliminating hepatitis C, patient care pathways must be simplified, including diagnostic approaches.
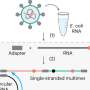
The standard approach to diagnosis of chronic hepatitis C infection is initial screening with an antibody test, followed by a laboratory-based molecular viral load testing, to confirm the presence of active virus and need for treatment. However, access to laboratory-based viral load tests remains limited in many LMICs. As a result, many of those with chronic hepatitis C are never linked to care and treatment.
Hepatitis C viral load testing performed on point-of-care devices outside of the laboratory and in a clinic near to where patients receive care are being increasingly used as an alternative approach to laboratory-based diagnosis. WHO already recommends the use of these point-of-care tests for diagnosis and monitoring of other infectious diseases, including tuberculosis, COVID-19 and HIV. Until recently, there had been limited data on their use in improving access to and uptake of hepatitis C testing and treatment.
More information: Adam Trickey et al, Impact of hepatitis C virus point-of-care RNA viral load testing compared with laboratory-based testing on uptake of RNA testing and treatment, and turnaround times: a systematic review and meta-analysis, The Lancet Gastroenterology & Hepatology (2023). DOI: 10.1016/S2468-1253(22)00346-6
Provided by University of Bristol