This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Regenerative drug restores bone in preclinical study
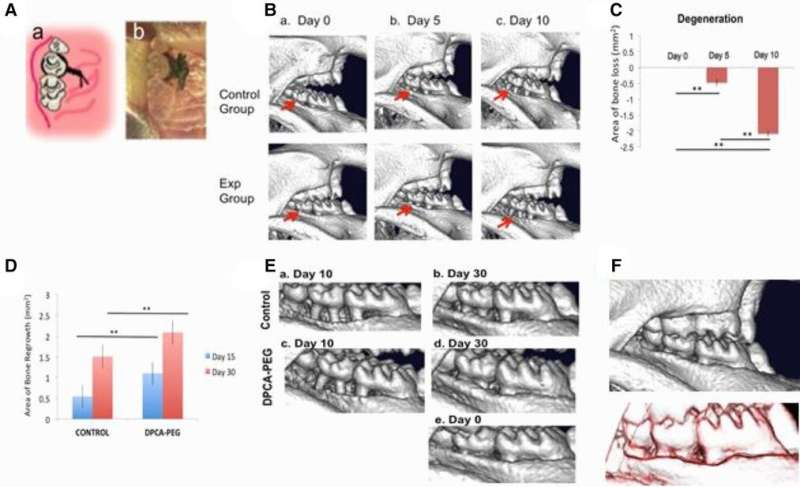
Bone loss is a part of aging that compromises quality of life and movement in many older people, but regenerative treatments to improve their health and well-being have been limited. Now, a study led by Lankenau Institute for Medical Research (LIMR) scientist Ellen Heber-Katz, Ph.D., has demonstrated the ability of an experimental regenerative medicine developed in her lab to restore bone in an animal.
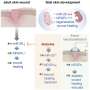
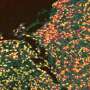
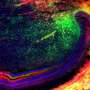
The preclinical study focused on a model of periodontal disease, which causes gum and bone loss that lead to tooth loss. In older individuals, the disease not only causes pain and discomfort but is the most common cause of tooth loss, affecting 30-60% of adults. However, study results showed that time release of the experimental drug, called 1,4-DPCA, fully restored diseased gums and the surrounding jaw bone, completely preventing tooth loss. Findings were published in November by the Heber-Katz team at LIMR, part of Main Line Health, in the journal Frontiers in Dental Medicine.
"Development of this experimental drug represents one of the most cutting-edge research directions LIMR has driven in the 21st century," said George Prendergast, President and CEO of the Institute. "In going beyond the uncertainties of stem cell treatments, this study also offers the first preclinical proof of concept for an off-the-shelf drug that could dramatically improve the health span of an individual by regenerating bone. It also for the first time suggests the potential anti-aging uses for this drug treatment as far as broadening how it can be used to instruct perfect healing by the body."
The periodontal disease treatment is one branch of Heber-Katz's research in regenerative medicine, a field that she pioneered in a shocking new direction in the mid-1990s. That's when she discovered a strain of laboratory mice that disproved the scientific assumption that only starfish and amphibians like salamanders were capable of healing wounds in a way that looked as if the injury never occurred.
"The results of this study are as powerful as we could have anticipated," said Heber-Katz, the Daniel B. and Florence E. Green Endowed Chair in Regenerative Medicine Research. "The restoration of significant amounts of lost bone and tissue was complete. I'm optimistic that this drug will move forward and eventually be used to prevent tooth loss in patients suffering from periodontal disease—one of the many ways we think it could be useful."
The drug 1,4-DPCA works by inhibiting a molecule that blocks production of a master molecule called hypoxia-inducible factor-1 (HIF-1a), a key component that is part of the body's healing response. By temporarily elevating HIF-1a, the drug shifts a tissue's metabolic state toward one used in early fetal development, where perfect healing without scarring is possible.
Heber-Katz came to LIMR, an organization with expertise in early drug discovery and development, to advance work on compounds like 1,4-DPCA to activate HIF-1a. The work was based on her earlier biological research on how tissue regeneration can be stimulated during the body's healing response to an injury.
Among Heber-Katz's fellow researchers were LIMR faculty members Azamat Aslanukov, Ph.D., and Kamila Bedelbaeva, MD, Ph.D.. Other collaborating researchers were from the University of Pennsylvania School of Dental Medicine and UC Berkeley.
Heber-Katz has two related patent-pending products that are nearing the stage of human testing:
- Medicines composed of 1,4-DPCA or related compounds that are formulated as topical or injectable hydrogels. These formulations are used to promote skin healing or restore skin integrity, including tissue damaged by natural aging. Chronic wounds often do not heal for older people
- Sutures infused with 1,4-DPCA to prevent scarring during the healing of surgical wounds
More information: Elan Zebrowitz et al, Prolyl-hydroxylase inhibitor-induced regeneration of alveolar bone and soft tissue in a mouse model of periodontitis through metabolic reprogramming, Frontiers in Dental Medicine (2022). DOI: 10.3389/fdmed.2022.992722