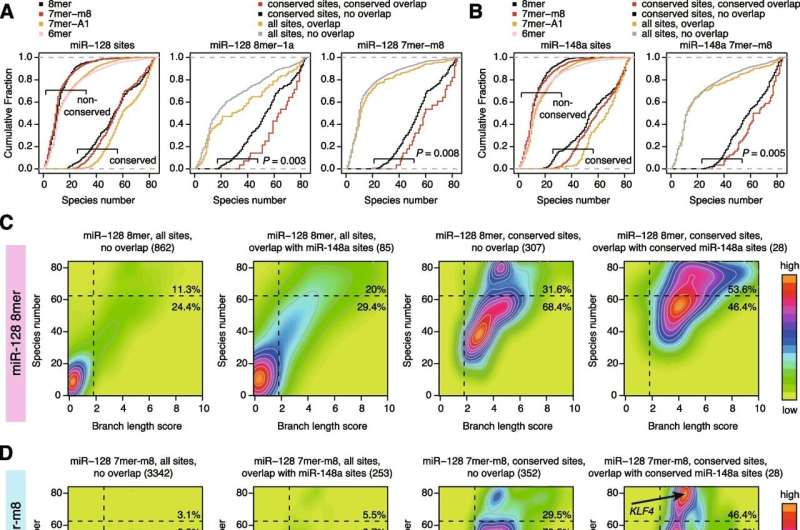
Conservation trends of miR-128 and miR-148a target sites with “conserved overlap.” A, B Cumulative distribution of the number of species in which the indicated site types of miR-128-3p (A) and miR-148a-3p (B) are conserved across 84 species. Conserved and non-conserved sites are defined by TargetScan. Target sites are classified according to Fig. 3A. P values were calculated by the one-tailed Wilcoxon rank sum test. C–E Density and contour plots showing the distribution of BLS values and the number of species in which sites are conserved. The results for all sites with no overlap, all sites with overlap, conserved sites with no overlap, and conserved sites with “conserved overlap” of miR-128-3p 8mer (C), miR-128-3p 7mer-m8 (D), and miR-148a-3p 7mer-m8 (E) are shown. Vertical and horizontal dashed lines indicate BLS cutoffs and the species number threshold (n = 62), respectively. Numbers in parentheses are the numbers of target sites. Credit: BMC Biology (2022). DOI: 10.1186/s12915-022-01447-4
A group of researchers from the Graduate School of Medicine at Nagoya University in Japan have discovered the impact of microRNA (miRNA) on inflammation in lupus in mice. They identified two miRNAs that are downregulated in the disease and an uncommon situation that occurs in which multiple miRNAs regulate the same set of genes.
Although the human body has many types of RNA, the most important is messenger RNA, which is involved in the creation of proteins in the body. The body also contains miRNA, which binds to regions of the messenger RNA to inhibit protein production and regulate several important bodily functions such as development, growth, and metabolism. Problems with miRNA are associated with several diseases including cancer and HIV. Now, the Nagoya University research group has identified the role of miRNA in systemic lupus erythematosus, a disease in which the human immune system attacks itself. They published their findings in BMC Biology.
Pairing with the correct messenger RNA target is determined by the "seed" of miRNA, a sequence that determines whether the miRNA can bind or not. The seed is like a "key" to the messenger RNA's "lock." However, this is complicated by the nature of miRNA's interaction with messenger RNA as a single particle of messenger RNA may be regulated by multiple miRNAs and the miRNA-messenger RNA pairs do not have to be an exact match to exert an effect.
As the effects of a single miRNA on a binding receptor site tend to be modest, stronger effects are often regulated by multiple miRNAs working in concert. This occurs through two processes. The first of these processes is "neighborhood" miRNA co-targeting, where two nearby miRNAs affect messenger RNA. The second, is "seed overlap" miRNA co-targeting, which is similar to the neighborhood type except both have similar nucleotides, so they bind to messenger RNA in such a way that some of their nucleotides overlap.
Given that altered miRNA expression has been reported in lupus disease, researchers have long suspected a connection. Now, a group of scientists, headed by Professor Hiroshi Suzuki at the Department of Molecular Oncology, and Lecturer Noritoshi Kato and Researcher Hiroki Kitai at the Department of Nephrology at the Nagoya University Graduate School of Medicine, have performed miRNA expression profiling using mice with lupus to investigate the role of miRNA in the disease.
The researchers found that two microRNAs, miR-128 and miR-148a, were down-regulated in plasmacytoid dendritic cells in lupus patients. As plasmacytoid dendritic cells play a crucial role in antiviral immunity and antibody production, they have been implicated in the initiation and development of several autoimmune and inflammatory diseases, including lupus. Both miR-128 and miR-148a target a gene called KLF4, which is associated with inflammatory control and the production of cytokines that regulate the activity of the immune system.
"Assuming that the expression levels of the other miRNA are maintained, the downregulation of one miRNA can be compensated for by the other microRNA," Suzuki explains. "However, when two miRNAs decrease simultaneously, as in lupus disease, alterations in their target—in this case KLF4—emerge."
One of the most important findings of the study was that as miR-128 and miR-148a share common nucleotides, they can bind to messenger RNA using "seed overlap" miRNA co-targeting. "miR-128 and miR-148a target KLF4 through extensive 'seed overlap' miRNA cotargeting. In this case, it negatively regulates the production of inflammatory cytokines," says Suzuki. "Therefore, this study collectively suggests the complexity of different modes of miRNA cotargeting and the importance of their perturbations in human diseases."
The researchers also performed integrative analyses, discovering that "seed overlap" miRNA cotargeting of KLF4 is a prevalent feature in other species. "We found that the conserved overlap site of KLF4 is the same in most species between humans and Coelacanths," said Suzuki. "Therefore, we expanded these findings by integratively analyzing seed overlap patterns of all miRNAs and the conservation patterns of 'seed overlap' target sites."
Suzuki and the research team discovered two main conservation classes of miRNA target sites. The first was shared by eutherian mammals, including animals that have a placenta. The second was shared by other animals, including humans and Coelacanths, and has a stronger association with both "seed overlap" and "neighborhood" miRNA cotargeting.
"Our study provides a comprehensive view of 'seed overlap' miRNA cotargeting, which is very important for the process by which lupus develops from the viewpoint of gene regulation and miRNA evolution. These findings highlight the importance of miRNA co-targeting in human pathology and the unique evolutionary aspects of miRNA co-targeting and miRNA target site conservation," Suzuki explains.
Suzuki also sees the potential of his research in treating lupus patients: "Testing for downregulation of the two miRNAs may help identify patients with high level of inflammation who may benefit from specific therapeutic development," he says.
More information: Hiroki Kitai et al, Systematic characterization of seed overlap microRNA cotargeting associated with lupus pathogenesis, BMC Biology (2022). DOI: 10.1186/s12915-022-01447-4
Journal information: BMC Biology
Provided by Nagoya University