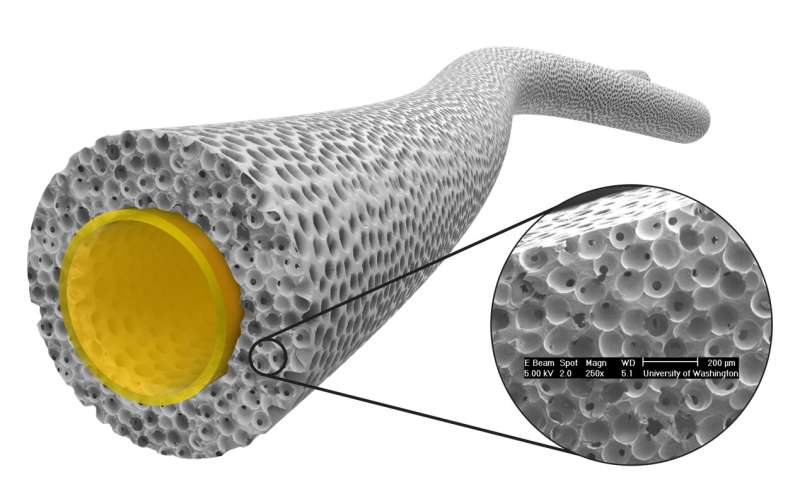
A pro-healing vascular graft under development at the University of Washington based upon a wall structure with 40 micron interconnected pores. Credit: Jeremy Baribeau
Blood vessel replacements (vascular grafts) are used today for hemodialysis blood access, trauma repair and cardiovascular reconstruction. The first synthetic vascular grafts (blood vessel replacements) were developed just after World War II and were fabricated from materials such as parachute cloth, stitched on home sewing machines. By the 1970s, commercial vascular grafts were introduced, made primarily from Dacron fabric or expanded Teflon (ePTFE).
These larger diameter (>5mm) grafts work satisfactorily for replacing or repairing large blood vessels. However, there are many needs where small diameter vascular grafts (SDVG) would be beneficial. For example, there are about 1 million leg amputations worldwide, mostly due to clogged blood vessels.
Synthetic blood vessel grafts in small sizes most often fail in this demanding, low-flow anatomical site. Also, surgeons would be pleased to have synthetic grafts suitable for heart bypass surgery, but no SDVG will work in this site. The FDA has never approved vascular grafts with diameters smaller than 5 mm. Why after 70+ years have vascular grafts not advanced in performance? There have been almost 50,000 technical papers published on vascular grafts, many proposing novel designs and novel biomaterials. So, research is ongoing—yet there is no cohesive theory to drive SDVG development.
In a perspective article published in the journal BME Frontiers, a new hypothesis is formulated that may reveal a path to the desired SDVG. After reviewing historical precedents and the pros and cons of many vascular graft approaches, the article proposes that we must emulate the structure and behavior of the natural, living artery.
Key elements of a next generation SDVG will be a living, functioning endothelial cell lining, a blood vessel network within the walls of the graft (a "pseudo vasa vasorum") and biomechanics to match the elasticity of the natural artery being replaced. Importantly, the graft cannot trigger the foreign body reaction (FBR) that leads to a fibrotic scar capsule with low vascularity and activated macrophages.
Most of our accepted biomaterials today (Teflon, Dacron, for example) trigger this FBR. The FBR reaction, if mild, is accepted by medical device regulatory agencies. However, the case is made that the FBR is really a manifestation of chronic inflammation—an ongoing, unending attack against the biomaterial.
The biomaterial essentially never truly heals in the body. Thus, so-called biocompatible biomaterials are perhaps not so biocompatible! This ongoing inflammation inhibits the formation of a healthy endothelial cell layer and the rigid foreign body capsule prevents the normal flexing pulsation of an implanted graft. Grafts showing the FBR are poised to fail because they do not have a healthy, living endothelial lining and their non-flexing walls and capsule contracture reduce blood flow and produce flow disturbances which lead to thrombosis.
Fortunately, there are new biomaterials being developed that inhibit the FBR and show reconstructive, regenerative healing. The central hypothesis of the paper suggests that these types of new materials can lead to successful vascular grafts that more closely emulate the performance of the natural artery. A number of vascular graft approaches based upon engineering natural tissues show this regenerative healing and are being explored in the lab and clinic.
These have promise, but there is a concern about long-term durability due to the body's mechanisms to digest older tissue. Also, there are new biomaterials such as a porous structure with 40 micron interconnected pores that heals in a non-fibrotic, vascularized manner. These new vascular graft approaches offer the possibility of SDVG that heal in a reconstructive manner and function much like the natural vessel they are intended to replace.
More information: Buddy Ratner, Vascular Grafts: Technology Success/Technology Failure, BME Frontiers (2022). DOI: 10.34133/bmef.0003
Provided by BMEF (BME Frontiers)