This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cocaine use disorder alters gene networks of neuroinflammation and neurotransmission in humans
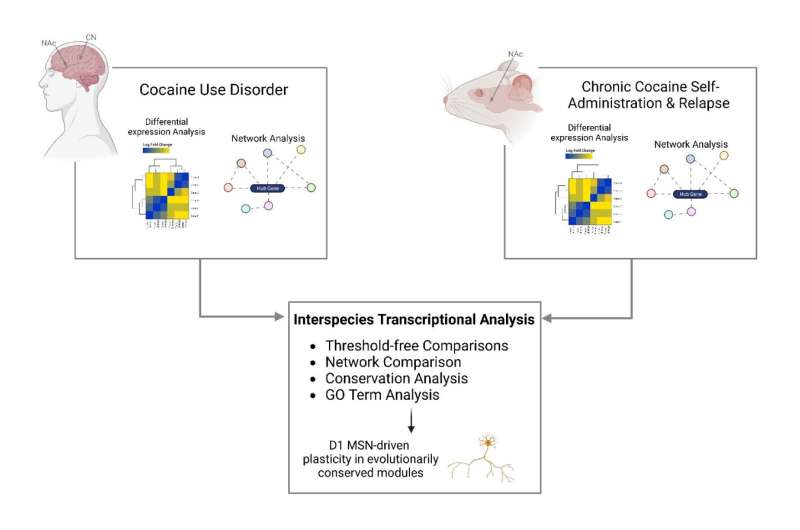
Individuals with cocaine use disorder exhibit gene expression changes in two brain regions: the nucleus accumbens, a region associated with reward, and the caudate nucleus, a region mediating habit formation, according to research conducted at the Icahn School of Medicine at Mount Sinai and published February 10 in Science Advances.
These changes, which contribute to the persistent behavioral abnormalities seen in addiction to drugs, occur because cocaine use sets off a series of chemical reactions that lead to increases in the amount of messenger RNA being produced from some of the affected genes in these two brain regions, whereas the activity of other genes decreases.
Changes in the amount of messenger RNA produced—a process also known as "expression" of the underlying genes—lead to changes in the amount of proteins that are produced and that subsequently carry out chemical reactions in the brain. The research team found a significant overlap between the RNAs expressed in these two brain regions, suggesting that these molecular changes may be key to the development and maintenance of cocaine use disorder.
Cocaine use disorder is a chronic, relapsing brain disorder for which there are currently no FDA-approved medication treatments. While it is hypothesized that regulation of gene expression in the brain's reward and motivational centers plays a critical role in the persistent behavioral changes that define addiction, knowledge remains limited of the maladaptive gene activity that chronic cocaine use causes in these circuits in humans and that underlies cocaine use disorder.
To address the knowledge gap, the research team performed RNA sequencing in both the nucleus accumbens and caudate nucleus from the postmortem brain tissue of persons with cocaine use disorder and matched controls. Using the largest and most diverse cohort examined to date, they found that neuroinflammatory processes are suppressed and that synaptic transmembrane transporters and ionotropic receptors—proteins that control how nerve cells communicate with one another in the brain—are enriched in the striatum of people with cocaine use disorder.
Cocaine increases the amount of the neurotransmitter dopamine at synapses, or junctions between two brain cells where electrical signals are converted into chemical signals. By doing so, the research team found, cocaine sets off a cascade of events that activate a chemical messenger in the brain called cyclic AMP, which then triggers changes in gene expression.
"In addition to the new insights into the molecular changes that cocaine use confers, we found that people with cocaine use disorder have dysregulated genes associated with schizophrenia and major depressive disorder, which indicates that these disorders may share some underlying gene regulatory and neural circuit systems," said Philipp Mews, Ph.D., Instructor of Neuroscience at Icahn Mount Sinai and first author of the paper together with Ashley M. Cunningham, a neuroscience Ph.D. student.
"Importantly, the transcriptional abnormalities—in particular, the neuroinflammatory responses that are suppressed in the nucleus accumbens of people with cocaine use disorder—are directionally opposite of the proinflammatory cascade responses conferred by opioid use disorder. The observation that there are distinct molecular changes conferred by each of the two substance use disorders could be valuable for the development of targeted, effective treatments specific to cocaine use disorder."
Because it is difficult to directly study how drugs like cocaine affect the human brain, researchers often use animal models to study their effects. However, a key question is whether what they learn from these animal models is similar to what happens in the brains of humans who use cocaine.
"Our research team looked at studies performed in mice that were given the opportunity to self-administer cocaine and compared the resulting molecular changes to those seen in postmortem brain tissue of people with cocaine use disorder. Our analysis revealed strikingly similar changes in the brain's gene expression profiles in both the mice and humans, validating the use of mouse models to study the pathophysiological basis of cocaine use disorder," said Eric J. Nestler, MD, Ph.D., Nash Family Professor of Neuroscience, Director of The Friedman Brain Institute, Dean for Academic Affairs at Icahn Mount Sinai, Chief Scientific Officer of the Mount Sinai Health System, and senior author of the paper.
"It is also important to emphasize that our human brain cohort includes a significant number of Black individuals, who have not been well represented in prior transcriptional studies of cocaine use disorder, despite longstanding evidence that the highest rate of overdose deaths involving cocaine is among Black individuals. Together, these findings represent a considerable advance in our understanding of the molecular abnormalities in cocaine use disorder and provide a highly valuable resource for future investigations."
More information: Convergent abnormalities in striatal gene networks in human cocaine use disorder and mouse cocaine administration models, Science Advances (2023).