This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New evidence on how individuals transition from recreational to compulsive drug use
While substance use disorder (SUD) remains a clinically and socially devastating condition in the U.S. and worldwide, the phenomenon of how individuals transition from recreational to compulsive drug use is yet to be fully understood.
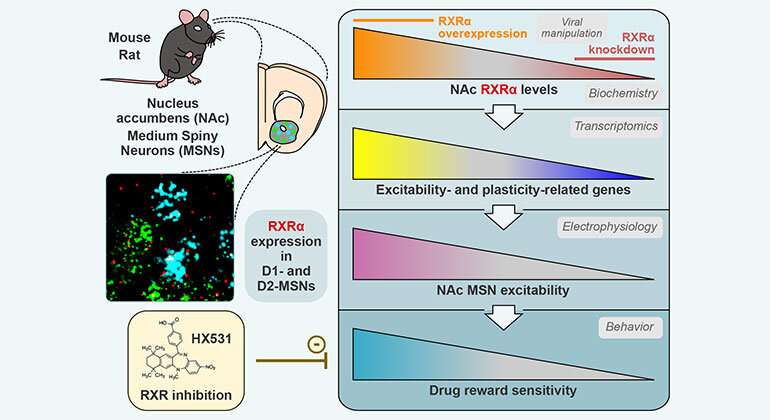
Researchers at the Icahn School of Medicine at Mount Sinai have opened the door to a promising new realm of discovery with the identification of a molecule and a previously unsuspected signaling pathway that appear to be pivotal to the pathophysiology of drug addiction. The molecule is known as Retinoid X Receptor Alpha (RXRα) in the nucleus accumbens, a key brain region for addiction, which appears to dictate in part the sensitivity of individuals to the initially rewarding properties of drug use. The team's findings were reported in the journal Neuron.
Specifically, the nucleus accumbens is a region of the brain's reward circuitry that integrates midbrain inputs of dopamine—a neurotransmitter that affects many aspects of behavior, including how we experience pleasure—to shape reward- and motivation-related behaviors.
Within this construct, researchers in the Nash Family Department of Neuroscience and the Friedman Brain Institute at Icahn Mount Sinai learned that RXRα positively correlated with addiction-relevant behavioral features in laboratory rodents—a finding that makes the molecule a strong mechanistic candidate to link individual vulnerability to cocaine addiction. Fundamental to the study was RNA sequencing on six regions of the rodent's brain reward circuitry after cocaine self-administration, withdrawal, and relapse in mice.
The discovery not only paves the way for future research on the novel signaling pathway, but on preventive strategies for drug addiction targeting RXRα. The Mount Sinai team showed that inhibition of the molecule using a systemically administered RXRα antagonist reduced cocaine-induced associative learning with tolerable side effects.
Scientists were particularly intrigued and encouraged by the fact that a single molecule could have such a profound effect on modulating complex behaviors related to drug addiction. It further highlighted for them the effectiveness of bioinformatically harnessing large-scale datasets as part of the effort to unravel novel answers to brain maladaptations in an unbiased manner.
Previous studies have linked RXRα signaling to other neuropsychiatric pathologies—including Alzheimer's disease, Parkinson's disease, multiple sclerosis, and diabetes—and Mount Sinai researchers believe that their latest findings could lead to future studies of RXRα involvement in psychiatric disease states like these.
Over the short term, they plan to apply their research to opioid drugs, whose rampant spread and abuse resulted in more than 564,000 deaths in the U.S. from overdose in the period 1999 to 2020, and for which early evidence suggests a similar action of RXRα.
More information: Arthur Godino et al, Transcriptional control of nucleus accumbens neuronal excitability by retinoid X receptor alpha tunes sensitivity to drug rewards, Neuron (2023). DOI: 10.1016/j.neuron.2023.02.013