This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Model illuminates environmental cues that may contribute to breast cancer recurrence

Nearly 270,000 people in the United States are diagnosed with breast cancer each year.
According to the Susan G. Komen Foundation, about 70%–80% of these individuals experience estrogen receptor-positive (ER+) breast cancer, where cancer cells need estrogen to grow. In terms of treatment, this presence of hormone receptors provides a nice handle for targeting tumors, say with therapies that knock out the tumor cell's ability to bind to estrogen and prevent remaining breast cancer cells from growing.
However, even if treated successfully, on average, one in five individuals with ER+ breast cancer experience a late recurrence when dormant tumor cells in distant parts of the body, such as the bone marrow, reactivate anywhere from five to over 20 years after initial treatment.
Researchers at the University of Delaware are advancing tools to better understand how this occurs.
In a new paper published in Science Advances on Wednesday, March 8, UD researchers led by chemical and biomolecular engineer April Kloxin report a new 3D model system for understanding cell-to-cell interactions between bone marrow cells and dormant breast cancer cells.
"The big problem we're trying to address is the need for improved human disease models," said Kloxin, Thomas and Kipp Gutshall Development Professor of Chemical and Biomolecular Engineering. "In this case, we're talking about breast cancer, specifically late recurrence that occurs at sites far away from the original tumor, which can be really difficult to detect in a timeframe that's useful for treating the patient before additional growth and spread of the cancer from those sites occurs.
"We wanted a better model system to study the mechanism of the recurrence and to evaluate therapeutic approaches to prevent late occurrences from happening."
Cell-to-cell communication
Kloxin explained that information in the literature and in her own studies support that the microenvironment which disseminated tumor cells experience in the body can influence whether breast cancer cells initially die, become dormant, or become dormant and then wake up.
Lina Pradhan, a postdoctoral researcher in the Kloxin Group and the paper's lead author, had observed in animal models that disseminated breast cancer cells were typically found situated near the bone lining. This made her wonder if these bone-forming cells played a role in promoting dormancy of breast cancer tumor cells.
"We observed dormant breast cancer cells near the bone line area in vivo, which made me think more about the importance of indirect interactions of individual cells for promoting dormancy," said Pradhan.
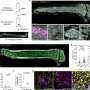
To explore this mechanism, the researchers developed a 3-dimensional system that allowed them to place the bone cells and tumor cells into two independent chambers separated by a membrane that only allowed secreted proteins to pass between them. The cells weren't touching each other or competing for space—real challenges that often occur when two cell types are cultured together in a two-dimensional system.
The unique 3D system allowed the researchers to watch as small proteins were exchanged between the breast cancer cells and the bone cells, and what they saw was interesting.
In the presence of bone cells, there were inflammatory-associated proteins that were produced. Some of the breast cancer cells died. However, more importantly, a significant percent of the breast cancer cells went into survival mode and began recycling material in the breast cancer cells, through a process called autophagy, that allowed the tumor cells to persist without growing.
Studying the cells in a three-dimensional, soft environment (hydrogel) that closely resembles tissues that the cell would experience in the body seems to be key.
"We think the context in which a cell is experiencing these different secreted proteins may matter to the cell response, in addition to factors intrinsic to the cells themselves, in terms of a tipping point for whether these inflammatory environments promote death or dormancy," Kloxin said.
Modeling these interactions can help scientists and engineers understand what's happening, and subsequently target new treatments or surveillance approaches for earlier detection of these secondary location recurrences. Separating the breast cancer cells from the bone cells in their 3D-model system allowed the research team to watch the breast cancer cells wake up, too, and begin to consider ways to stop them from awakening and causing cancer recurrence.
The research team has protected the model system with a provisional patent through the University's Office of Economic Innovation and Partnerships (OEIP). Kloxin said she hopes the team's model system can be useful to others working on improved treatment strategies or to better study how these cells reactivate and then use therapeutics to suppress this reactivation behavior.
Kloxin knows firsthand how hard it is to watch a loved one experience cancer recurrence. Her mother, Helen Morris, was diagnosed with estrogen receptor-postive breast cancer in 2003. Morris underwent successful treatment and remained cancer-free for 17 years, until she experienced a late recurrence in 2020. Kloxin, too, is a breast cancer survivor.
Because the model's synthetic matrix is robust and reproducible, the researchers believe it also could help scientists studying other diseases or cancers.
"We hope that our model system can be one little piece that is helpful in looking for solutions for long-term cancer recurrence," said Kloxin.
Future work in the Kloxin lab could include partnerships on patient-derived cell studies to explore what drives tumor cell progression. The research team also is collaborating with Inventia to road test the biotechnology company's bioprinting platform for translating the team's 3D culture models for breast cancer and wound-healing technologies, among other things.
More information: Lina Pradhan et al, Dynamic bioinspired coculture model for probing ER + breast cancer dormancy in the bone marrow niche, Science Advances (2023). DOI: 10.1126/sciadv.ade3186