Credit: Circulation Research (2023). DOI: 10.1161/CIRCRESAHA.122.321583
Greater awareness and advances in treatment have greatly improved survival rates following heart attack. With more survivors, however, has come the challenge of managing long-term impacts on heart function, especially chronic heart failure, in which the heart gradually loses its ability to pump blood.
Mortality among individuals affected by chronic heart failure following a heart attack—referred to medically as myocardial infarction (MI)—is high. But, according to new research from a major collaborative effort led by scientists at the Lewis Katz School of Medicine at Temple University, more effective treatments may soon be within reach.
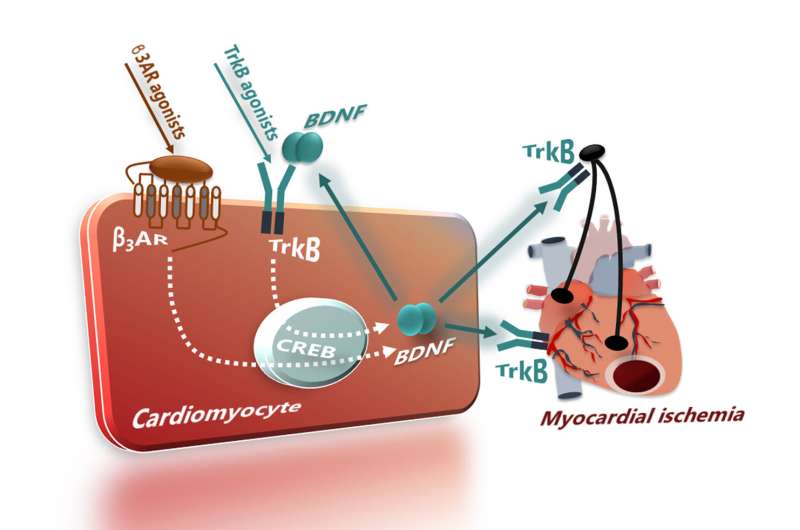
The new work centers on a molecule known as brain-derived neurotrophic factor (BDNF), showing that the loss of BNDF underlies post-MI heart failure. More significantly, the researchers demonstrate in animals that BDNF levels can be replenished therapeutically—abolishing damage to heart tissue and enabling the heart to recover.
"The identification of BDNF as a therapeutic target is an important step forward in the development of improved treatments for heart failure," said Walter J. Koch, Ph.D., W.W. Smith Endowed Chair in Cardiovascular Medicine, Professor and Chair of the Department of Pharmacology and Director of the Center for Translational Medicine at the Katz School of Medicine, and senior author on the new study.
In addition to delineating the role of BDNF, Dr. Koch and colleagues show for the first time that reduced BDNF generation in the heart is associated with altered signaling of the β-adrenergic receptor (βAR) and that stimulation of a protein known as tropomyosin kinase receptor B (TrkB) enriches BDNF levels in the heart. "These actions combined attenuate the progression of heart failure," Dr. Koch said.
Researchers Nazareno Paolocci, MD, Ph.D., Associate Professor of Medicine at Johns Hopkins University School of Medicine, and Alessandro Cannavo, Ph.D., a former postdoctoral fellow in Dr. Koch's laboratory at Temple and now Associate Professor at University of Naples Federico II in Italy and lead author on the new report, collaborated with Dr. Koch to carry out the study. The group's findings were published by the journal Circulation Research.
The researchers began their investigation by examining BDNF levels in wild-type mice whose hearts had been temporarily starved of blood, producing a state known as ischemia and thereby mimicking the effects of a heart attack. The mice subsequently developed chronic heart failure and were then treated with a TrkB agonist. In another set of experiments, the researchers generated mice that lacked BDNF expression in the heart. These BDNF knockout mice were also subjected to ischemia, similar to wild-type mice.
Knowing that β3AR stimulation can also enrich BDNF in cells, the researchers further investigated whether the effects of TrkB were linked to βAR signaling. "Drugs that act on βAR protect patients against heart attack, but the mechanism that the drugs use to exert their actions has been unknown," Dr. Koch explained.
The experiments revealed post-ischemic changes in BNDF levels, characterized initially by an increase in BDNF, followed by a steep decline, and showed that TrkB stimulation restored BDNF levels and improved post-ischemic heart function. These effects were bolstered by BDNF enrichment in heart cells driven by a β3AR, a distinct βAR subtype. "BDNF generation is another mechanism of defense put forth by heart cells to protect themselves against ischemia," Dr. Paolocci remarked.
Although further research is needed to better understand whether TrkB and β3AR interact to generate BDNF, the findings could have immediate translational implications. "Selective stimulation of β3AR by existing agonists could readily be adapted for use in patients with chronic heart failure," Dr. Koch noted. "Future treatments may also leverage TrkB, which is a promising target for the development of new heart failure therapies."
More information: Alessandro Cannavo et al, β3AR-Dependent Brain-Derived Neurotrophic Factor (BDNF) Generation Limits Chronic Postischemic Heart Failure, Circulation Research (2023). DOI: 10.1161/CIRCRESAHA.122.321583
Journal information: Circulation Research
Provided by Temple University