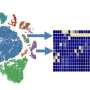
Integrated metabolomics and transcriptomics data analysis of human kidney samples. a, Total of 50 human kidney samples were collected for metabolomics analysis; including healthy controls (n = 25) and patients with KD (n = 25). b, Volcano plot of metabolites showing significant changes in human diseased kidneys. The x axis shows log2 fold change (log2FC). The y axis shows −log10(P value). Color indicates metabolites significantly higher (red) or lower (blue) in human diseased kidneys. Welch’s two-sided t-test was used to calculate P value. c, Metabolic pathways showing significant changes in diseased kidneys. The dot color indicates the level of significance, the dot size indicates pathway impact. P value was calculated from the enrichment analysis in MetaboAnalyst. d, The simplified NAD salvage pathway. Blue indicates metabolites significantly lower in human diseased kidneys. e, The levels of NAD+, NMN, NR and NAM in human kidneys (healthy control n = 25 and KD n = 25). *P < 0.05. NS, not significant. Data are presented as mean ± s.e.m. and were analyzed using Welch’s two-sided t-test. f, The bulk RNA-seq of same human kidney samples used for metabolomic studies. g, Gene ontology analysis of cellular composition of genes significantly correlating with kidney NAD+ levels. The dot color and size indicate significance and gene counts, respectively. h, The correlation between kidney NAD+ levels (x axis) and relative genes expression (y axis) encoding mitochondrial proteins. P value was calculated using Pearson’s correlation. Credit: Nature Metabolism (2023). DOI: 10.1038/s42255-023-00761-7
Roughly one million people die of untreated kidney failure, worldwide, each year. Despite the major personal and economic burden, only a few new approaches have been deployed to treat or cure kidney disease over the last 40 years.
Metabolic changes related to an enzyme "helper molecule"—called nicotinamide adenine dinucleotide (NAD)—may serve as the basis for a future treatment or preventive measure for kidney disease, according to a discovery by a new study led by researches in the Perelman School of Medicine at the University of Pennsylvania.
This work was published in Nature Metabolism.
Metabolic changes related to NAD may offer a new therapeutic target to improve the course of kidney disease. By mapping metabolite changes in healthy and diseased mouse and human kidneys, the Penn scientists consistently identified differences in levels between healthy and diseased kidneys, including a prominent decrease in NAD in those that were diseased.
But when mice in the study were provided with an over-the-counter supplement, it was shown to be effective in reversing NAD loss. This indicates that NAD could play an important role in protecting from kidney dysfunction.
"We hope that this research can lead to improved care in the future. So when patients have metabolite changes, they can receive treatment before kidney disorders arise," said co-lead investigator Katalin Susztak, MD, Ph.D., a professor of Nephrology and member of the Institute for Diabetes, Obesity, and Metabolism (IDOM) and Kidney Innovation Center at Penn Medicine.
Susztak and her colleagues—including co-lead investigator Joseph Baur, Ph.D., a professor of Physiology at Penn and member of the IDOM and first author Tomohito Doke, MD, Ph.D., a postdoctoral fellow in Susztak's lab—are working to fill a gap in research. Prior to this study, samples from human patients have not been used in metabolomic studies, meaning the studies of small molecules (like metabolites), in relation to kidney disease.
Using unbiased metabolomic studies, the team identified changes in NAD metabolism in both mouse and human kidneys. In the mouse studies, they showed that using a common supplement, nicotinamide riboside or nicotinamide mononucleotide, to boost NAD protected mice from kidney dysfunction by protecting the mitochondria—the powerhouses—of kidney tubule cells.
Kidney tubule cells are used to return critical filtered nutrients to the body's blood stream. And when the mitochondria in those cells are damaged, the researchers found, a pathway causing inflammation and kidney disease development is activated. By preventing the damage to mitochondria, NAD supplements suppressed this inflammation and protected mice from kidney injury.
"Identifying these downstream mechanisms that are sensitive to NAD is critical to understanding which conditions may benefit from NAD supplementation," said Baur.
With these findings, the study team hopes that their research will serve as a springboard for further studies into the role of metabolite changes in kidney dysfunction, as well as the development of new pharmaceuticals to prevent and treat kidney disease.
More information: Joseph Baur, NAD+ precursor supplementation prevents mtRNA/RIG-I-dependent inflammation during kidney injury, Nature Metabolism (2023). DOI: 10.1038/s42255-023-00761-7. www.nature.com/articles/s42255-023-00761-7
Journal information: Nature Metabolism