This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
Conservative Texas judge weighs challenge to abortion pills
A conservative federal judge in Texas heard arguments Wednesday from a Christian group seeking to overturn the Food and Drug Administration's more than 2-decade-old approval of an abortion medication, in a case that could threaten the most common form of abortion in the U.S.
Lawyers for the Alliance for Defending Freedom asked Judge Matthew Kacsmaryk during the hearing in Amarillo, Texas, to issue an immediate order that would revoke or suspend the drug mifepristone's approval. Such a step would be an unprecedented challenge to the FDA, which approved mifepristone in combination with a second pill as a safe and effective method for ending abortion in 2000.
During a 90-minute presentation to the court, alliance attorney Erik Baptist told the judge that removing mifepristone from the market "would restore proper policing power to the states"—a reference to last summer's U.S. Supreme Court ruling that overturned Roe v. Wade and left it to states to decide on the legality of abortion.
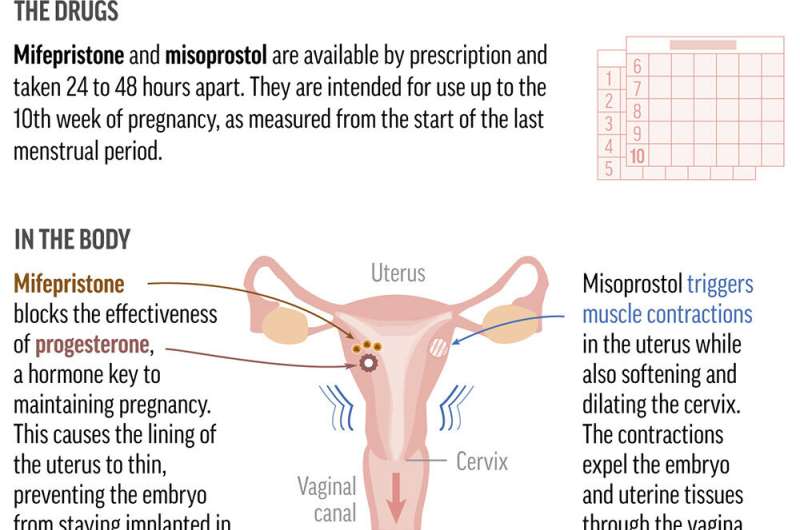
Mifepristone, when combined with a second pill, has become the most common method of abortion in the U.S. and has been increasingly prescribed since Roe was overturned.
Acknowledging the significance of the case, Kacsmaryk, who was appointed by then-President Donald Trump, asked Baptist if he could cite a prior example of a court removing an FDA-approved drug after many years on the market.
Baptist acknowledged that there are no prior examples, but he blamed the drug's longevity on the FDA's "stonewalling" of his group's prior requests to remove the drug. The group petitioned the FDA in 2002 and in 2019 seeking to curb access to the pill.
Lawyers for the FDA are expected to argue that pulling mifepristone would upend reproductive care for U.S. women and undermine the government's scientific oversight of prescription drugs.
Kacsmaryk gave each side two hours to make their arguments—with time for rebuttal—in the high-stakes case. Mifepristone's manufacturer, Danco Laboratories, will join the FDA in arguing to keep the pill available.
A ruling could come any time after arguments conclude. A decision against the drug would be swiftly appealed by U.S. Department of Justice attorneys representing the FDA, who would also likely seek an emergency stay to stop it from taking effect while the case proceeds.
One of the alliance's chief arguments against the FDA is that it misused its authorities when it originally approved the pill.
The FDA reviewed the drug under its so-called accelerated approval program, which was created in the early 1990s to speed access to the first HIV drugs. Since then, it's been used to expedite drugs for cancer and other "serious or life-threatening diseases."
The alliance, which was also involved in the lawsuit that led the Supreme Court to overturn Roe, argues that pregnancy is not a disease and therefore mifepristone should not have been considered for accelerated approval.
"The contrast between these illnesses and the FDA jamming pregnancy into ... the FDA regulations could not be more stark," Baptist told Kacsmaryk.
But the FDA says the group's argument is flawed on multiple counts. First, FDA regulations make clear that pregnancy is considered a "medical condition" that can be serious and life-threatening in some cases.

Second, while the FDA reviewed the drug under its accelerated approval regime, it didn't expedite the drug's review. In fact, approval only came after four years of deliberation. Instead, the FDA used regulatory powers under the accelerated program to add extra safety restrictions to mifepristone, including requiring physicians to be certified before prescribing it.
The hearing is the first in the case and is being closely watched by groups on both sides of the abortion issue in light of the reversal of Roe.. Removing mifepristone from the market would curtail access to abortion even in states where it's legal.
If Kacsmaryk rules against the FDA, it's unclear how quickly access to mifepristone could be curtailed or how the process would work. The FDA has its own procedures for revoking drug approvals that involve public hearings and scientific deliberations, which can take months or years.
If mifepristone is sidelined, clinics and doctors that prescribe the combination say they would switch to using only misoprostol, the other drug used in the two-drug combination. That single-drug approach has a slightly lower rate of effectiveness in ending pregnancies but is widely used in countries where mifepristone is illegal or unavailable.
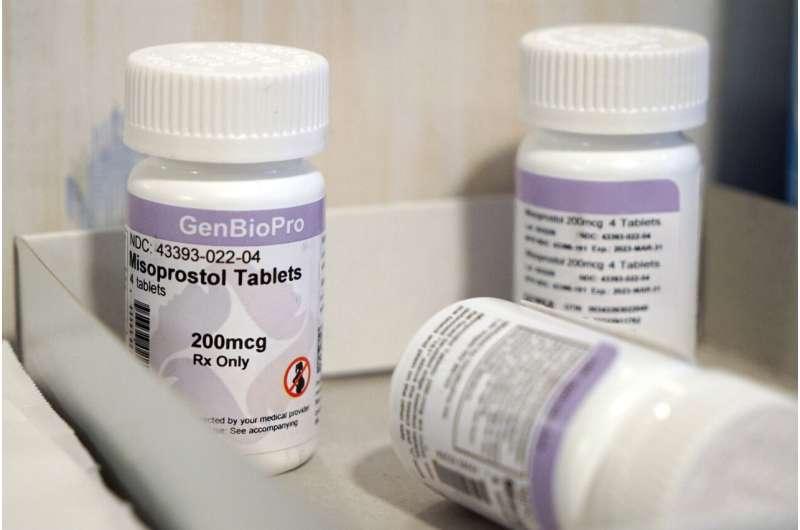
In addition to challenging mifepristone's approval process, the lawsuit takes aim at several later FDA decisions that loosened restrictions on the pill, including eliminating a requirement that women pick it up in person.
Lawyers for the FDA have pointed out that serious side effects with mifepristone are rare, and the agency has repeatedly affirmed the drug's safety by reviewing subsequent studies and data. Pulling the drug more than 20 years after approval would be "extraordinary and unprecedented," the government stated in its legal response.
© 2023 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed without permission.