This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers develop a universal oral COVID-19 vaccine that prevents severe illness in hamsters
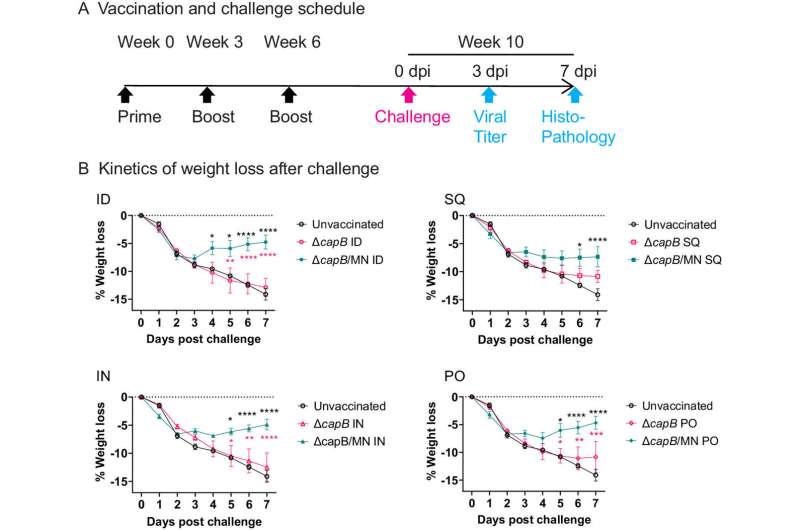
A UCLA-led team has developed an inexpensive, universal oral COVID-19 vaccine that prevented severe respiratory illness and weight loss when tested in hamsters, which are naturally susceptible to SARS-CoV-2. It proved as effective as vaccines administered by injection or intranasally in the research.
If ultimately approved for human use, it could be a weapon against all COVID-19 variants and boost uptake, particularly in low- and middle-income countries, and among those with an aversion to needles.
The study is published in the journal Microbiology Spectrum.
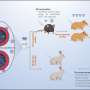
The oral vaccine is based primarily on the nucleocapsid protein, which is the most abundantly expressed of the virus's four major structural proteins and evolves at a much slower rate than the frequently mutating spike protein. The vaccine utilizes a highly weakened bacterium to produce the nucleocapsid protein in infected cells as well as the membrane protein, which is another highly abundant viral structural protein.
"Being a universal vaccine based primarily upon the nucleocapsid protein, the vaccine is resistant to the incessant mutations of the SARS-CoV-2 spike protein upon which virtually all current vaccines are based," said senior author Dr. Marcus Horwitz, distinguished professor of medicine in the Division of Infectious Diseases and of microbiology, immunology and molecular genetics at the David Geffen School of Medicine at UCLA. "As a result, current vaccines rapidly become obsolete, requiring that they repeatedly be re-engineered. Hence, our vaccine should protect against new and emerging variants of SARS-CoV-2."
Oral delivery also makes it easier to distribute the vaccine in resource poor areas of the world by eliminating the need for needles, syringes, and trained personnel to deliver injectable vaccines, he added. "An oral vaccine may also be attractive to many people with vaccine hesitancy on account of fear of needles."
The researchers noted that while it worked exceptionally well in preventing severe respiratory illness, it did not provide full protection against high viral loads in the hamsters. Also, they did not test it against the omicron strain, which contains a nearly identical nucleocapsid protein, because of this strain's low virulence in the golden Syrian hamsters they used.
But the vaccine, they write, "is efficacious when administered via the oral route against COVID-19-like disease in a highly demanding animal model. This conveniently administered, easily manufactured, inexpensive, and readily stored and transported vaccine could play a major role in ending the COVID-19 pandemic by protecting immunized individuals from serious disease from current and future strains of SARS-CoV-2."
The next step in the process will be to manufacture the vaccine for oral administration via an acid-resistant enteric capsule that will allow the vaccine to be safely released in the small intestine, Horwitz said. It will then be tested for safety, immunogenicity, and efficacy in humans.
"We also plan to expand the vaccine to protect against infections caused by other types of potentially pandemic coronaviruses such as the virus that causes Middle Eastern Respiratory Syndrome (MERS)," he added.
More information: Qingmei Jia et al, Oral Administration of Universal Bacterium-Vectored Nucleocapsid-Expressing COVID-19 Vaccine is Efficacious in Hamsters, Microbiology Spectrum (2023). DOI: 10.1128/spectrum.05035-22