This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Bioengineering a better adeno-associated virus vector for gene therapy
A new study characterizes a bioengineered adeno-associated virus (AAV)3B capsid variant that demonstrates improved transduction to human liver cells. Another advantage of the AAV3B-V04 capsid was its significantly reduced seroreactivity to human serum samples, as reported in the study published in the peer-reviewed journal Human Gene Therapy.
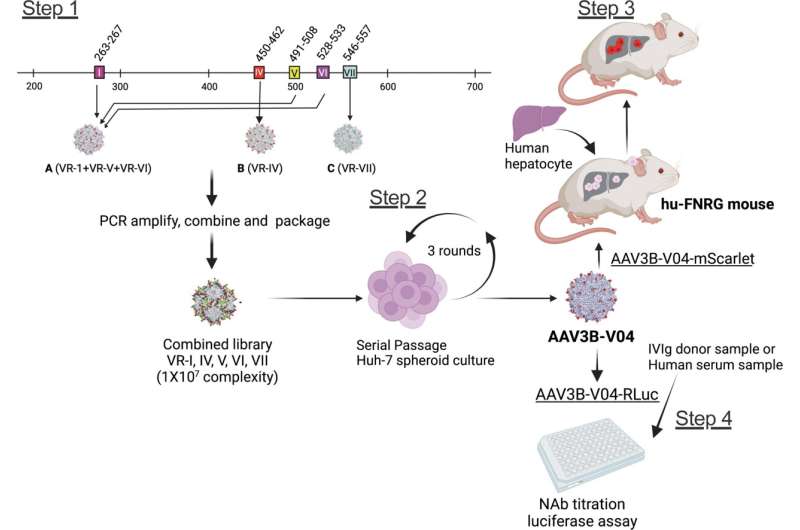
Moanaro Biswas, from Indiana University School of Medicine, and co-authors, generated an AAV3B combinatorial targeted variant library and used directed evolution to isolate enhanced liver targeting variants. Hepatic gene therapy with AAV vectors offers the potential for long-lived correction of monogenic disorders. One of the variants generated, AAV3B-V04, was shown to have significantly improved tropism for human hepatocytes.
The presence of pre-existing neutralizing antibodies (NAb) to AAV capsids can trigger an immune response, which restricts patient eligibility for gene therapy. The investigators found that 44% of human serum samples with pre-existing NAbs to AAV3B had 5-20-fold lower reciprocal NAb titers to AAV3B-V04.
"We conclude that the candidate variant AAV3B-V04 described here is superior to its parent AAV3B capsid," state the investigators. "AAV3B-V04 provides distinct advantages such as improved transduction efficiency, tissue tropism and reduced immunogenicity."
"The liver remains a primary target for gene therapy, but the utility of AAV-based gene therapy is still limited by the relatively high doses of vector required and by pre-existing immune responses," says Editor-in-Chief Terence R. Flotte, MD, Celia and Isaac Haidak Professor of Medical Education and Dean, Provost, and Executive Deputy Chancellor, University of Massachusetts Chan Medical School. "Any new AAV capsid that can improve the potency of the vector and allow it to evade pre-existing serum antibodies could be highly significant."
More information: Jyoti Rana et al, Characterization of a Bioengineered AAV3B Capsid Variant with Enhanced Hepatocyte Tropism and Immune Evasion, Human Gene Therapy (2023). DOI: 10.1089/hum.2022.176