This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Important role of intestinal immune cells in iron deficiency identified for the first time
Iron deficiency is one of the five main causes of impaired health. It affects 30 percent of the world's population, particularly women. Why iron deficiency can occur, even if enough iron is supplied through the diet, has not yet been sufficiently clarified in scientific research.
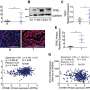
For the first time, a research team from MedUni Vienna has discovered that certain immune cells in the intestine play an important role in iron absorption in the body. The study results may provide a new approach for possible therapeutic measures and were recently published in the journal Blood.
Approximately one to two milligrams of the trace element must be supplied daily through food and finally absorbed in the duodenum for a balanced iron metabolism. For the first time, the research team led by Nyamdelger Sukhbaatar and Thomas Weichhart from MedUni Vienna's Centre for Pathobiochemistry and Genetics has now shown that certain immune cells (macrophages) in this section of the intestine control iron absorption.
Specifically, the research revealed that the activation of macrophages directly in the duodenum leads to a halt in iron availability in the body.
"We were able to determine that the macrophages in the duodenum eat away the iron transport molecule transferrin, so to speak. This means that the iron remains in the intestinal cells and can no longer enter the bloodstream," explains first author Nyamdelger Sukhbaatar. In addition, the study found that macrophages are also activated during fasting, food intake or during an intestinal infection, thereby changing the amount of transferrin in the intestine.
"Our findings thus represent a real paradigm shift, as it was previously assumed that transferrin is always present in equal amounts everywhere in the body and does not actually play any role in iron regulation," underlines study leader Thomas Weichhart.
New approach for therapeutic options
Against the background of their study results, the research team is currently investigating whether the macrophages in the intestine and their regulation of transferrin could also be disturbed in inflammatory bowel diseases, intestinal infections or gastritis.
Potential therapeutic approaches already exist: In animal models, clinically approved drugs (mTOR inhibitors or serine protease blockers) were able to increase the amounts of transferrin and restore iron availability for the organism. Whether or not these treatment options can also be used in humans is to be researched in further studies as well.
Balanced iron metabolism is important for health
A balanced iron metabolism is an essential prerequisite for health. Iron is an important component of the blood pigment hemoglobin, which is responsible for transporting oxygen in the red blood cells. If the body lacks this trace element, anemia is the result. Equally fatal is an excess of iron triggered by certain genetic diseases such as haemochromatosis, whereby the excess iron deposition destroys many organs in the long term.
Therefore, our organism has developed some, partly redundant mechanisms to absorb the precise amount of iron. Nonetheless, the most common causes of iron deficiency and anemia include not only iron-deficient nutrition, but also impaired iron absorption despite sufficient availability of iron in the diet. The new study suggests that immune cells in the duodenum might be responsible for iron absorption problems.
More information: Nyamdelger Sukhbaatar et al, Duodenal macrophages control dietary iron absorption via local degradation of transferrin, Blood (2023). DOI: 10.1182/blood.2022016632