This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Organoids shown to speed glycoengineered vaccine development
Testing the efficacy of a vaccine candidate is typically a long process, with the immune response of an animal model taking around two months.
A multi-institution team, led by Matt DeLisa, the William L. Lewis Professor in the Smith School of Chemical Biomolecular Engineering, at Cornell Engineering, is developing a method that is more than an order of magnitude faster.
Using a biomaterials-based organoid, developed in the lab of former Cornell professor Ankur Singh, now at the Woodruff School of Mechanical Engineering at the Georgia Institute of Technology, the team was able to assess the strength of the immune response in just days.
"It's about being able to interrogate much larger numbers of vaccine candidates in a single experiment, and to do it much faster," DeLisa said. "We can profile the potency of dozens or even hundreds of subunit vaccine antigens at a time, and the immunological responses to each can be observed in as little as four days."
DeLisa and Singh are co-senior authors of "Profiling Germinal Center-like B Cell Responses to Conjugate Vaccines Using Synthetic Immune Organoids," which published in ACS Central Science.
Co-lead authors are Tyler Moeller, Ph.D. '21, a microbiologist in the U.S. Navy; and Shivem Shah, Ph.D. '20, now a student at Columbia University School of Medicine.
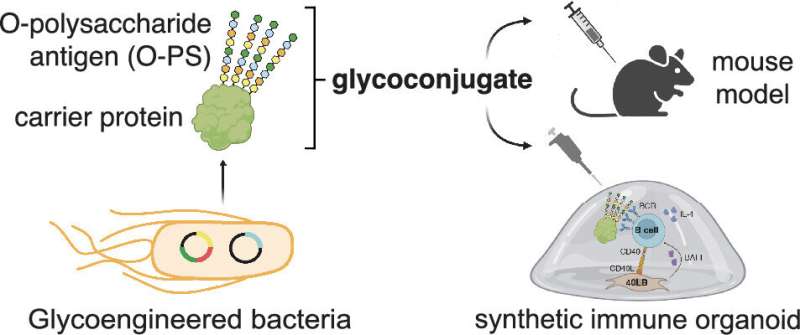
One of the focal points of DeLisa's lab is a particular type of vaccine known as a glycoconjugate, which trains the immune system to respond to specific polysaccharides that are found on the exterior of pathogenic bacteria or malignant cells. Although all cells in nature are decorated with many different polysaccharides on their surfaces, glycoconjugates are custom engineered to target unique polysaccharides that are only present on a pathogenic bacterium or cancer cell.
"The design and engineering of glycoconjugates against different disease targets is an important part of vaccinology," he said. "In the case of pathogenic bacteria, glycoconjugate vaccines typically target lipid-associated polysaccharides known as capsular polysaccharides or lipopolysaccharides, which can be thought of as biomolecular fingerprints—with each type of pathogen displaying a distinct polysaccharide fingerprint on its surface."
These outer surface polysaccharides represent an "Achilles heel" that can render a pathogen susceptible to glycoconjugate vaccines that target its carbohydrate structures, DeLisa said.
Vaccines promote B cell receptor clustering in germinal centers, which are formed when the vaccine initiates the production of antibodies from B cells. These important germinal center responses are difficult to study in living organisms such as mice. To get around this issue, Singh's lab has developed laboratory models of the immune system based on organoid cultures that make it easier to investigate the intricacies of immune responses.
Instead of having to use mouse models to characterize the potency of their glycoconjugates—and only being able to conduct a single test per animal—the researchers' protocol uses tissue from a single mouse to create hundreds of immune organoids, each of which can be used to assess a different vaccine candidate.
"Let's say we make hundreds of these vaccine candidates, because glycoengineered vaccines can be assembled in a variety of ways," Singh said. "It is practically impossible—from a personnel standpoint, animal-use standpoint and financially—to have thousands of animal models for the studies. Here, from a single spleen of a mouse, we can make hundreds of these organoids, which can then be used to transform preliminary vaccine testing into a streamlined process that takes only a few days to complete."
Germinal center B cells recapitulate many aspects of the natural immune response, including production of mutant antibody clones that have a broad range of affinities for the immunizing antigen as the researchers showed. The beauty of this method, Singh said, is the ability to also discover high-affinity antibodies that have specificity for the antigen—in this case either the polysaccharide component or the protein component—all using a single mouse tissue.
"Nobody has ever done it," he said. "The ability to look for high-affinity clones from the organoid, and really come up with the best quality antibody candidates in such a short period of time, could be a game-changer."
For this work, the team tested just two glycoconjugate vaccine candidates—one known to be a strong immunogen, and one known to be weaker. The organoid assessments confirmed each candidate's relative strength.
"The organoid B cell biomarkers that we had identified were, in fact, predictive of the stronger and weaker immunogenicity of our two test candidates," DeLisa said. "So we think all the pieces are now in place for a follow-on study, in which immune organoids are used for screening a large library of glycoconjugate variants to uncover the most potent designs, which we're now working on."
Further testing will involve larger numbers and different types of vaccine candidates, and more investigation into the specific immune response and production of antibodies elicited by different vaccine types.
More information: Tyler D. Moeller et al, Profiling Germinal Center-like B Cell Responses to Conjugate Vaccines Using Synthetic Immune Organoids, ACS Central Science (2023). DOI: 10.1021/acscentsci.2c01473