This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Two studies find role for alternative lengthening of telomeres in treatment-resistant tumors
Two recent papers from researchers in the Children's Hospital of Philadelphia (CHOP) Cancer Center have advanced the understanding of treatment-resistant pediatric tumors, both in assessing a novel treatment and in characterizing the role of alternative lengthening of telomeres (ALT) in these refractory tumors.
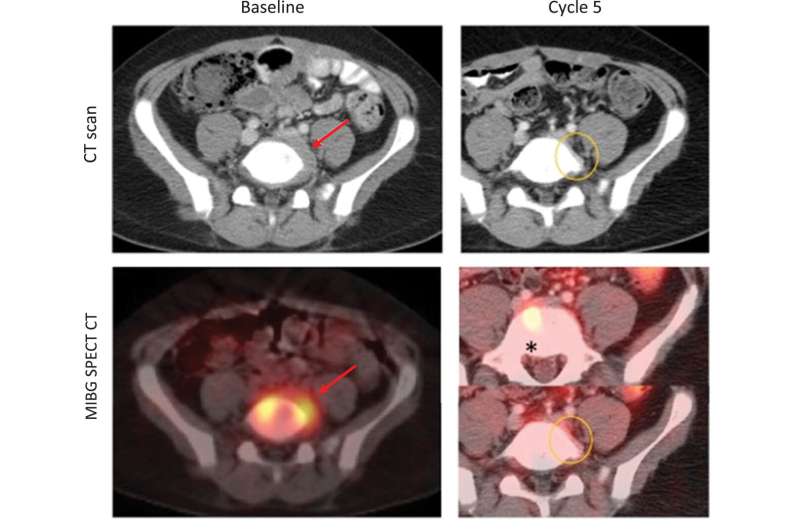
The first paper, published recently in the journal Cancer, provided data from a phase 1/2 Children's Oncology Group (COG) clinical trial led by CHOP researchers that investigated the use of a combined therapy of adavosertib and irinotecan for relapsed neuroblastoma, medulloblastoma and rhabdomyosarcoma. Adavosertib is a cell cycle checkpoint inhibitor that blocks the tyrosine kinase WEE1, which is involved in DNA replication, and by doing so sensitizes tumor cells to cytotoxic agents and replication stress. The trial combined adavosertib with irinotecan, with the aim of enhancing tumor shrinkage and allowing for a prolonged exposure to the treatments.
Although only the neuroblastoma cohort met the protocol defined efficacy endpoint, the results were the first positive signal of clinical activity in pediatric oncology for a small molecule inhibitor of the DNA damage repair and cell cycle checkpoint. The neuroblastoma group had an estimated 16.7% objective response rate.
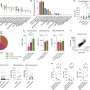
As part of the trial, the researchers explored potential predictors of response to treatment. One predictor they focused on was whether the tumors being treated had alternative lengthening of telomeres (ALT), a phenomenon that allows tumors to replicate uncontrollably; whereas telomeres in normal cells shorten as cells divide, the telomeres in some tumors lengthen, allowing cells to become effectively immortal. ALT is found in tumors of older pediatric and adolescent patients with neuroblastoma, and preclinical laboratory studies had suggested that ALT tumors may be sensitive to irinotecan and/or adavosertib.
Using a novel research test involving single cell immunofluorescence, the researchers found that 25% of patients whose tumors had ultrabright telomeres—indicating ALT—demonstrated tumor shrinkage when taking adavosertib and irinotecan, while no patients (0 of 4) whose tumors were not ultrabright had shrinkage. This finding requires further study as the cohort size was small and tumor tissue was not available from all enrolled on the trial.
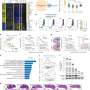
The second study, published in Neuro-Oncology, sought to validate the ALT test used in the Cancer study in tumor tissues. With the help of the Children's Brain Tumor Network (CBTN) and CHOP's Center for Single Cell Biology, the researchers used laboratory and computational approaches to catalog ALT in approximately 900 pediatric brain tumors.
Among 579 pediatric brain tumors that had corresponding whole genome sequencing through the Open Pediatric Brain Tumor Atlas (OpenPBTA), the researchers detected ALT in 6.9% of these tumors. Using the same assay as the Cancer paper, they completed additional validation by ultrabright telomeric foci in situ on a subset of these tumors.
The researchers confirmed that ALT is common (38.1%) in pediatric high-grade glioma (pHGG), but uncommon in other pediatric brain tumors (<3%). Importantly, the researchers found that mutations in ATRX, a gene associated with ALT, occurs in only 50% of ALT-positive pHGG, suggesting other mechanisms or alterations might lead to the development of ALT in these patients.
"In the future, ALT as tested by routine clinical sequencing may be a predictive biomarker to match effective treatments for ALT tumors," said Kristina A. Cole, MD, Ph.D., a pediatric neuro-oncologist at Children's Hospital of Philadelphia and senior author of both papers. "This approach may be useful in future studies that investigate whether WEE1 inhibitors or other agents that target replicative stress could help treat neuroblastoma and other ALT-activated tumors."
More information: Kristina A. Cole et al, Pediatric phase 2 trial of a WEE1 inhibitor, adavosertib (AZD1775), and irinotecan for relapsed neuroblastoma, medulloblastoma, and rhabdomyosarcoma, Cancer (2023). DOI: 10.1002/cncr.34786
Jennifer L Stundon et al, Alternative lengthening of telomeres (ALT) in pediatric high-grade gliomas can occur without ATRX mutation and is enriched in patients with pathogenic germline mismatch repair (MMR) variants, Neuro-Oncology (2022). DOI: 10.1093/neuonc/noac278