This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
The world of diabetes brought to life on a diminutive chip
Diabetes mellitus is a group of metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. The chronic hyperglycemia associated with diabetes is not only inherently dangerous, but it also can lead to various complications, raising serious concerns. Diabetes may be generally categorized as either type 1 (T1D), which arises from defects in insulin-secreting cells that result in the absence of insulin production, and type 2 (T2D), which stems from reduced insulin secretion due to unhealthy lifestyle habits such as poor diet and lack of exercise.
The Korean Diabetes Union reported that approximately 4.7 million Koreans were diagnosed with diabetes in 2021, with over 90 percent suffering from T2D. The World Health Organization and the International Diabetes Federation predict that the global diabetes population will reach 300 million by 2025. Thus, diabetes is a worldwide issue, and many countries are vigorously researching treatments for the disease.
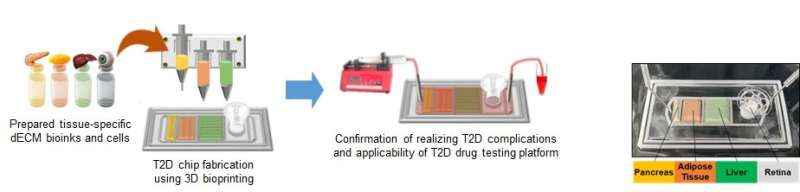
A team of researchers led by Professor Dong-Woo Cho, Dr. Ju Young Park and Ph.D. candidate Joeng Ju Kim from the Department of Mechanical Engineering at POSTECH and Professor Jae Yon Won from the Department of Ophthalmology and Visual Science at Eunpyeong St. Mary's Hospital of the Catholic University of Korea, have completed development of a multi-organ chip on-a-chip that applies 3D cell printing technology to closely replicate the pathological environment of T2D. The team's research findings were recently published in Advanced Functional Materials.
To replicate the pathological features of diabetes and its implications, the research team developed bioinks derived from the pancreas, adipose tissue, and liver, which are closely related to T2D. They then employed 3D cell printing to create a multi-organ chip on-a-chip with the most appropriate arrangement for reproducing the pathological characteristics of T2D. The bioinks were used to encapsulate cells from each organ and create an artificial tissue, allowing the chip to replicate the complex cellular interactions that occur in the human body.
The team experimented with the chip to examine the relationship between various adipose tissues and T2D, finding that the use of bioink derived from visceral adipose tissue clearly showed a correlation between the disease and visceral fat, which is more closely related than subcutaneous adipose tissue. Additionally, the addition of a retina compartment to the chip resulted in impaired retina cell function, indicating that the chip can also be used for researching diabetes complications.
The developed multiple-organ on-a-chip can be used to verify the efficacy of diabetes treatments currently in clinical trials. In fact, the device has already been proven to be about as effective as animal and clinical trials are, indicating that chip-based research may soon replace the need for animal and clinical testing.
More information: Joeng Ju Kim et al, Pathophysiological Reconstruction of a Tissue‐Specific Multiple‐Organ On‐A‐Chip for Type 2 Diabetes Emulation using 3D Cell Printing, Advanced Functional Materials (2023). DOI: 10.1002/adfm.202213649