May 16, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Bioengineering active immunotherapy for personalized cancer treatment
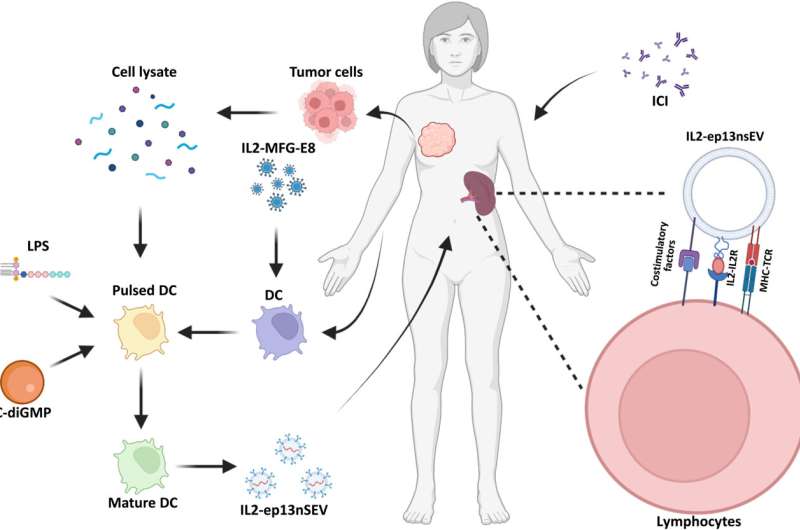
Breast cancer is resistant to immunotherapies; therefore, bioengineers and oncologists seek to develop a slew of therapeutic strategies to overcome this challenge. In a new report in Science Advances, Kerui Wu and colleagues in the departments of cancer biology, translation biology and breast surgery in the U.S., and China engineered active immunotherapy to create smart nanovesicles for personalized treatment. The research team accomplished this by anchoring membrane-bound bioactive protein interleukin-2 (IL2; made by a type of T lymphocyte) to maintain enriching and T-cell promoting co-stimulatory factors on dendritic cell-derived small extracellular vesicle surfaces.
The nanovesicles displayed major-hiscompatibility complex-bound antigens from dendritic immune cells. Upon administration, they saw how the surface-bound IL2 guided nanovesicles toward lymphoid organs associated with immune cells to activate similar immune receptors on lymphocytes. The vesicles named "IL2-ep13nsEV" induced a strong immune reaction in vivo to rescue approximately 50% of mice implanted with patient-derived xenografts while sensitizing cancer cells to immune checkpoint inhibitor treatment to prevent tumor recurrence. The outcomes present a feasible strategy to treat and prevent metastatic breast cancer in preclinical models with applications across diverse cancer types.
Nanoengineered smart vesicles
Clinical oncologists and bioengineers seek to introduce several new immunotherapeutic agents to improve the clinical outcome of specific cancers. For example, monoclonal antibody-based immune checkpoint inhibitors are markedly efficient to treat patients with lung cancer, melanoma and leukemia. Most patients are, however, resistant to immune checkpoint inhibitor therapies, where acquired resistance and relapse are common upon follow-up treatment.
Cancer resistance can result from low mutation burden; therefore, inhibitors are designed to enhance the anti-tumor function of T-cells, alongside a variety of active immunotherapies to generate tumor-targeted immune cells. However, the translational impact of such therapies including engineered chimeric antigen T cells can lead to life-threatening symptoms due to the controlled release of inflammatory cytokines.
Breast cancer cells are more resistant to immunotherapy due to its low mutation burden when compared to other cancer types. Wu and colleagues implemented immunotherapy through lipopolysaccharides and stimulator interferon gene agonist to promote the expression of a variety of co-stimulators on nanovesicle surfaces to generate enhanced active immunotherapy. The team loaded the vesicle with major histocompatibility complex presenting antigens derived from tumor lysates, alongside bioactive IL-2, and enriched costimulatory factors. This nanoengineered smart vesicle sought target lymphocytes and T-cells for personalized treatment for managing breast cancer.
Engineering co-stimulatory factors on the smart nanovesicle
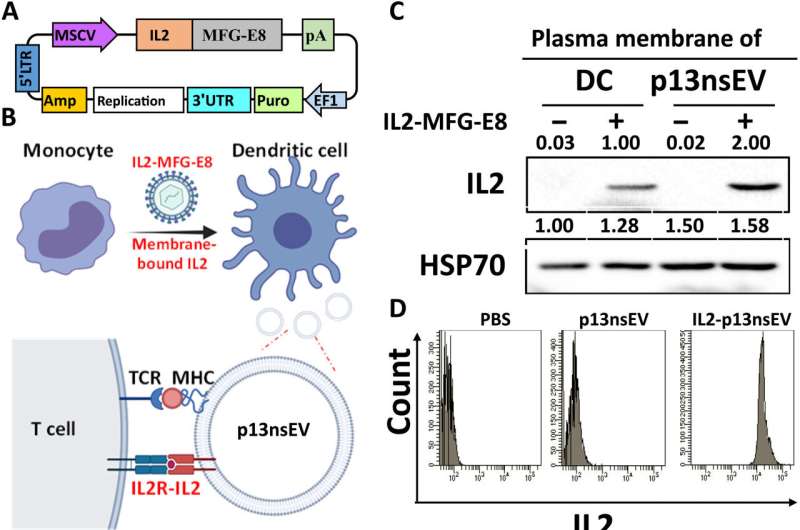
Wu and colleagues developed active immunotherapy by engineering natural nanovesicles secreted by dendritic cells to induce specific antitumor effects via lymphocyte activation in an immune-suppressive environment. The dendritic cell-derived nanovesicles maintained essential functionality to induce immunity. The team isolated the nanovesicles from a mouse dendritic cell line and from human primary cell lines to bioengineer and name them as "p13nsEV" first, followed by "IL2-ep13nsEV" during the study. They verified the viability of the constructs and purified them, followed by electron microscopy to visualize saucer-shaped proteins expressing major hiscompatibility loci I and II on the surfaces.
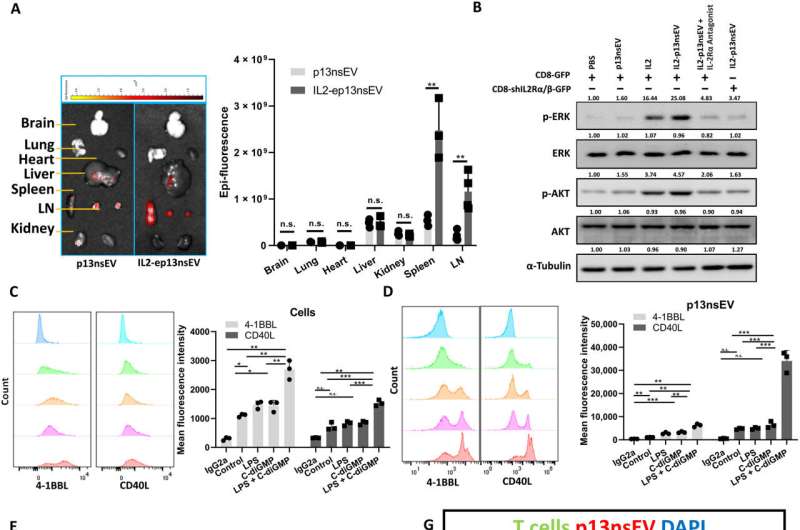
The scientists injected the nanovesicles to mice via the tail base and imaged them across various harvested organs to analyze their uptake. Wu and the team noted significant infiltration of the constructs in secondary lymphoid organs to indicate nanovesicles carrying neoantigens to immune cells for wide-spread circulation and infiltration of other immune organs.
Effects of the nanovesicles on breast tumor growth in a mouse model
The research team tested the ability of bioengineered nanovesicles to enhance cancer cell-specific killing of cytotoxic T lymphocytes in a mouse model and tracked the tumor growth of mice treated with such constructs. By four weeks they noticed the lowest tumor burden in mice treated with nanovesicles. The researchers tested if these constructs could mobilize T cells into tumor lesions and observed an increase in the natural killer cells in treated mice. They noted the significance of nanovesicle interactions with specific T cells such as CD 4 and CD 8 to suppress cancer.
The nanovesicles can work together with immune checkpoint inhibitors to enhance the effect of the latter strategy, during breast cancer treatment. While nanovesicles enable tumor growth suppression in immune-cold mouse models, it benefited the method of immune checkpoint inhibition. The two methods were complementary to rally a reactive immune response. For example, while nanovesicles generated cancer-cell specific lymphocytes, the immune checkpoint inhibitors maintained their viability to effectively eliminate cancer cells altogether. The combination of both methods increased the number of tumor-infiltrating lymphocytes and active T cells in the spleen compared to either technique alone.
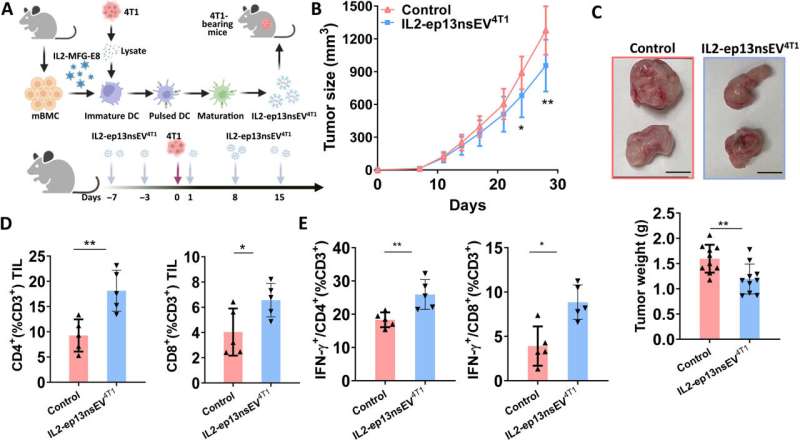
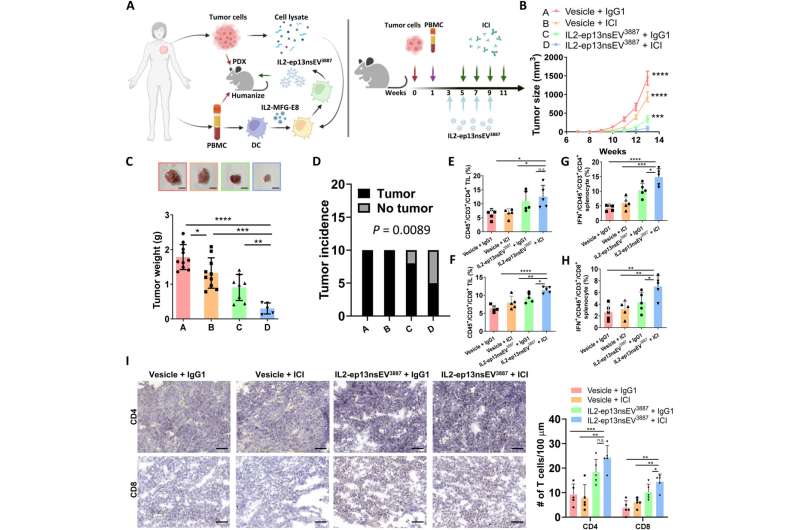
Preclinical investigations with a humanized patient-derived xenograft
Wu and colleagues next used a patient-derived xenograft to test the anti-tumor effect of nanoconstructs and combined the method against immune checkpoint inhibition as before. They noted the combined technologies to be more effective at tumor growth inhibition in humanized mice. The histochemistry outcomes showed how active immunotherapy vesicle treatment promoted increased T lymphocyte infiltration into the tumor.
While immune checkpoint inhibitors are administered in patients with advanced stage breast cancer, most patients undergo surgery as a curable approach at early stages. Nevertheless, patients are sometimes faced with recurrence, which leads to breast cancer–related death.
In such scenarios, Wu and colleagues recommend personalized active immunotherapy with nanovesicles to prevent future disease recurrence. They tested this theory on a mouse model and found the personalized treatment strategy could significantly decrease disease recurrence. They did not observe side-effects of the drug after 8-weeks of administration on the body weight, liver function or immune-cell hyperactivation in animals.
Outlook
In this way, Kerui Wu and the research team studied breast cancer intervention in the lab; a type of cancer with highest incidence in the United States. Although non-metastatic cancers can be treated with surgery and chemotherapy, approximately 22% of patients with breast cancer eventually experience recurrence within 10 years.
Most existing therapies fall behind in saving patients with a 10-year survival rate by about 13%. As a result, the need for better therapy is imperative to treat patients at advanced stages and prevent the recurrence of disease. The outcomes of the study are promising for treating multiple cancer types alongside tumors that are resistant to the method of immune checkpoint inhibition.
More information: Kerui Wu et al, Engineering an active immunotherapy for personalized cancer treatment and prevention of recurrence, Science Advances (2023). DOI: 10.1126/sciadv.ade0625
Wouter Scheper et al, Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers, Nature Medicine (2018). DOI: 10.1038/s41591-018-0266-5
© 2023 Science X Network