This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
GPR141 regulates breast cancer progression via oncogenic mediators and the p-mTOR/p53 axis: Study
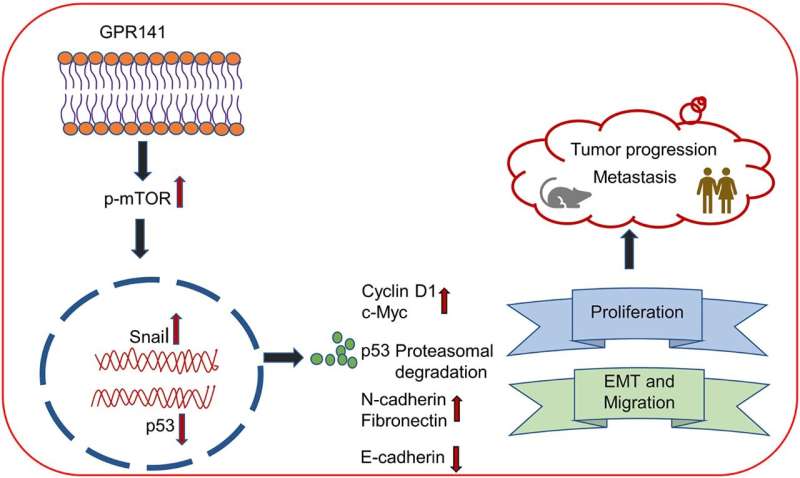
A new research paper was published in Oncotarget, titled "G-protein-coupled receptor 141 mediates breast cancer proliferation and metastasis by regulating oncogenic mediators and the p-mTOR/p53 axis."
Breast cancer morbidity is surging towards the peak in females across the globe. An inherent property of cancer cells is enhanced cell proliferation rate and migration capability, leading to deregulated cell signaling cascades. G-protein-coupled receptors (GPCRs) have recently emerged as a hot-spot target in cancer research.
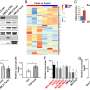
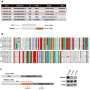
Researchers Monalisa Parija, Amit K. Adhya and Sandip K. Mishra from the Institute of Life Sciences, Regional Centre for Biotechnology and All India Institute of Medical Sciences identified aberrant expression of G-protein-coupled receptor 141 (GPR141) in different breast cancer subtypes that correlate with poor prognosis.
However, the molecular mechanism via which GPR141 advances breast cancer remains elusive. Increased GPR141 expression enhances the migratory behavior of breast cancer, driving oncogenic pathways both in vitro and in vivo through activation of epithelial to mesenchymal transition (EMT), oncogenic mediators and regulation of p-mTOR/p53 signaling.
"Our study unveils a molecular mechanism for p53 downregulation and activation of p-mTOR1 and its substrates in GPR141 overexpressed cells, accelerating breast tumorigenesis," note the authors.
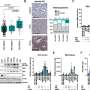
In their current study, the researchers found that an E3 ubiquitin ligase, Cullin1, partly mediates p53 degradation via proteasomal pathway. Co-immunoprecipitation results show that the phosphorylated form of 40S ribosome protein S6 (ps6., a p-mTOR1 substrate) forms a complex with Cullin1. These findings suggest an interplay between Cullin1 and p-mTOR1 in GPR141 overexpressed cells that downregulates p53 expression, thus inducing tumor growth.
GPR141 silencing restores p53 expression and attenuates p-mTOR1 signaling events, thereby impeding proliferation and migration in breast cancer cells. Their findings describe the role of GPR141 in breast cancer proliferation, and metastasis, as well as in influencing the tumor microenvironment. Modulating GPR141 expression could pave the way for a better therapeutic approach to regulating breast cancer progression and metastasis.
"In conclusion, our research highlights the gain of function of GPR141 drives breast tumorigenesis by inducing tumor cell properties via the p-mTOR1/p53 axis, altering EMT markers, and enhancing oncogenic mediators," say the researchers.
More information: Monalisa Parija et al, G-protein-coupled receptor 141 mediates breast cancer proliferation and metastasis by regulating oncogenic mediators and the p-mTOR/p53 axis, Oncotarget (2023). DOI: 10.18632/oncotarget.28433